From July 23rd to August 3rd, I was at Stanford from the Cardiothoracic Surgical Skills Summer Internship. It was a fascinating experience, combining surgical lab work with porcine hearts with informative anatomy lessons and lectures about cardiac surgery from various doctors. In regards to the lectures, we heard from Dr. James Fann about cardiac valve disease, Dr. Pat Kasinpila about cardiothoracic emergencies and trauma, and Dr. Mary Shui about the diagnostic window through the eye. All were extremely informative and taught me, as well as all of the other interns, a lot about cardiac surgery, from where it began to where it will be.
Cardiac Surgery
Dr. James Fann is a professor of cardiothoracic surgery at Stanford University, and he spoke about cardiac valve diseases. He first went into the anatomy of the mitral valve, which is the valve between the left atrium and left ventricle. The mitral valve, named after its visual similarity to a bishop’s miter, has two leaflets (anterior leaflet and posterior leaflet) that are connected to the heart muscle by a ring of tissue called the annulus. The chordae tendineae, or heart cords, keep the valve in place and connect to the papillary muscles, which when interacting with the left ventricle, close and open the valve. The mitral valve is connected to the aortic valve via collagen tissue, which is known as the fibrous skeleton of the heart. The aortic valve, which is the valve between the left ventricle and aorta, has three leaflets (left coronary cusp, right coronary cusp, and the non-coronary cusp). Both can undergo stenosis, a narrowing of the valve which prevents blood flow, or regurgitation, which detrimentally allows backward blood flow. Stenosis can be caused by rheumatic fever, calcification, neoplasm (tumors), endocarditis (infection of the inner layer or the heart), or just a congenital heart defect. Regurgitation can be caused by many of the same factors that cause valvular stenosis as well as ruptured chordate tendineae and enlarged fatty leaflets that allow prolapse.
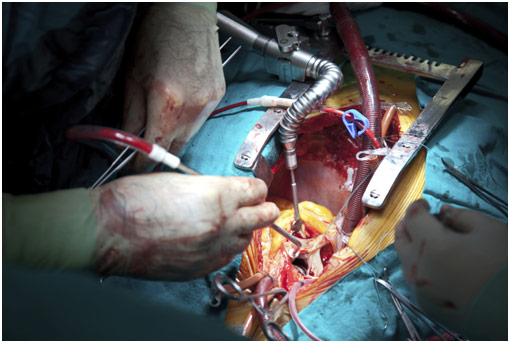
For either condition, an option is valve replacement surgery. Dr. Fann summarized the whole surgical process in 7 steps. First, there is the incision to gain access to the heart. Second, the patient goes on cardiopulmonary bypass. Third, the aorta is excluded or cross-clamped. Fourth, cardioplegia, a potassium solution to stop the heart, is given to protect the heart. The fifth is the valve replacement or repair itself. The sixth is removing the patient from cardiopulmonary bypass. Seventh is doing the closure of the incision made.
Prior to replacing the valve, one of the most important steps is picking out the proper valve. The options are a mechanical valve that lasts very long but requires blood thinners and a tissue valve that doesn’t last super long but does not require blood thinners. Thus, the valve must be chosen by examining various factors. Can the patient take blood thinners reliably? Is the patient going to do drugs that would change the efficacy of the blood thinners? Does the patient want to give birth in the future because blood thinners result in birth defects? Based on these factors among many more, the surgeon and the patient make a decision, and often, it is for a tissue valve, which is generally made from the bovine pericardium. This is partly because the risk of death for reoperation is about 3-5% for tissue valves compared to the 2% risk of stroke or major bleeding from blood thinners each year for mechanical valves.
In regards to how the surgeon can actually repair the valve, Dr. Fann talked about various transcatheter methods for stenosis, which are becoming increasingly common. One type is a percutaneous balloon valvuloplasty for stenosis. A catheter is sent to the aorta via the femoral artery or the femoral vein. From the femoral artery, it goes through the aorta, allowing access to either the aortic or mitral valve. Once access is ensured, a balloon-tipped catheter is sent to the valve where it can inflate to open up the stenosed valve. From the femoral vein, the catheter is passed across the atrial septum into the left atrium where is passed through the mitral valve before the balloon valvuloplasty is performed. This is done under rapid ventricular pacing, which is when the surgeons increase the heart rate of the patient dramatically so that blood pressure drops really low since the heart does not have enough time to fill with blood. Since this operation would not permanently fix the problem, the valve can also be replaced in what is called transcatheter aortic valve replacement. In a similar fashion, a catheter is put inside the heart near the valve. You then insert a valve-inside-a-stent, which crushes the old valve after the balloon is inflated (similar to a balloon valvuloplasty), allowing the new valve to take its place. Each of these ways represents an exciting transcatheter treatment option for valvular stenosis.
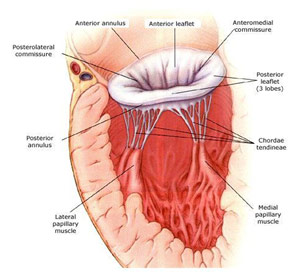
Mitral Valve Anatomy
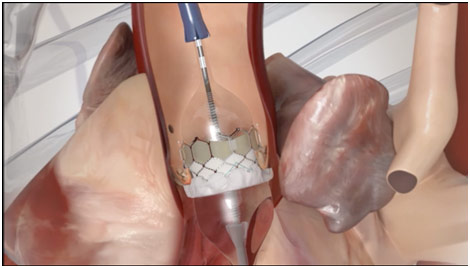
Balloon Inflating with Valve and Stent in TAVR
Dr. Pat Kasinpila is a cardiothoracic resident at Stanford, currently doing a research fellowship, and she spoke about cardiothoracic emergencies and trauma. She first began by discussing that trauma is any acute potentially life-threatening injury and how trauma is the leading cause of death for people who are younger than 44 years old. Trauma includes all kinds of injuries but can be usually split into two types, blunt injuries like motor vehicle and bicycle accidents and penetrating injuries like stabbings and gunshot wounds. The most important job in the trauma bay is to stabilize the patient and find out what’s wrong. Stabilizing the patient usually is done by following the ABCs, which is an acronym for airway, breathing, and cardiac system. They are specifically in that order based on what would kill the patient first. Thus, the airway is the most important because if the patient does not have a clear airway, they cannot get oxygen and will die in six minutes. The airway can be blocked by tracheobronchial injuries like smoke inhalation, chemical burns, and lacerations; head injuries; and maxillofacial traumas among other injuries. Breathing is the next most important, and it is important to note that it is not the same thing as the airway since a person can be breathing but not getting any oxygen to the lungs. Breathing issues can be caused by spinal injuries, broken ribs, pneumothorax (collapsed lung), pulmonary contusion (bruised lung), and flail chest (multiple breaks in contiguous ribs which immobilizes a section of the ribs). The last important one is circulation, which consists of the heart and other vital signs. The main factor is shock, which is a sudden loss of blood flow. Shock can be classified as neurogenic where a brain injury causes blood vessels to be especially dilated, septic where an infection also causes perfused vasodilation, hemorrhagic shock where excessive bleeding (internal or external) occurs, or cardiogenic shock where the heart does not get enough blood due to tension pneumothorax, cardiac tamponade, or an embolism. With a shock, the first vital sign to change is generally heart rate followed by blood temperature followed by skin temperature followed by the level of consciousness.
Dr. Kasinpila then talked about specific cardiothoracic emergencies and traumas that occur frequently in the emergency department. Myocardial contusion (bruised heart) was a big one that is often caused by blunt or high-impact accidents. Myocardial contusion presents very similarly to a heart attack with a troponin rise, chest pain, and an ST elevation. Sometimes also in the case of an MVA (motor vehicle accident), the doctor may also see a “seatbelt sign” where the seat belt stopped the patient from flying forward. Patients also tend to present with sternal fractures and rib fractures from the trauma. The standard of care is to do an EKG and echocardiography along with checking the cardiac enzymes. However, treatment only consists of stabilizing everything around the heart and providing supportive care to help the patient heal on themselves. Dr. Kasinpila then talked about hemothorax and pneumothorax, which are usually caused due to blunt or penetrating trauma which can cause issues from lung lacerations or rib fractures. Pneumothorax and hemothorax are differentiated since the former involves the leaking of air into the pleural space (membrane around the lungs) while the latter involves the leaking of blood into the lungs. They can cause a collapsed lung and even respiratory failure as the lungs are compressed by fluid. Diagnosis is usually done with a chest X-ray or sometimes a CT scan. The procedure is done on an emergency basis and involves inserting a chest tube into the patient between the 4th and 5th intercostal space. The chest tube goes into the pleura and stays there, draining all the fluid out of the pleural space, allowing the lung to re-expand.
Dr. Kasinpila went on to talk about cardiac tamponade, which is when blood, or more generally fluid, in the pericardium (sac around the heart) results in a decreased filling of the heart, so the heart starts to collapse and fail. What was especially surprising was that it took only 100 mL of blood to cause cardiac tamponade. While there are numerous causes for cardiac tamponade, generally it is caused by penetrating trauma like gunshot wounds or stab wounds. The patient usually presents with Beck’s triad, which includes hypotension, distended (enlarged) neck veins, and muffled heart sounds. Diagnosis of cardiac tamponade also needs ultrasound as well as this clinical diagnosis after which emergent pericardiocentesis is performed where a drain is left in the pericardium to remove the fluid and blood is transfused to the patient. She followed the discussion of cardiac tamponade with on aortic injuries, which is when there is a transaction, laceration, tear, or rupture of the aorta, usually in the ligament arteriosum. Generally, this is due to preexisting conditions like hypertension or Marfan syndrome among other causes; however, an aortic dissection can also be caused due to trauma. If there is a large rupture, the patient will usually die on the scene; however, in the case of a contained rupture where the outer layer is intact, the patient can survive with a procedure or operation. If there is no leaking or rupture, the patient can be taken to the catheter lab where a doctor can put a stent into the damaged section of the aorta and carefully manage the patient. The stent allows the blood to exclude the weakened section of the aorta, helping to avoid it from rupturing. In more extreme cases or when putting a stent is not an option, the aorta can instead be resected and replaced with a Dacron graft. Working in the trauma bay can be especially difficult due to the high mortality rate, but Dr. Kasinpila told us that it is also especially rewarding.
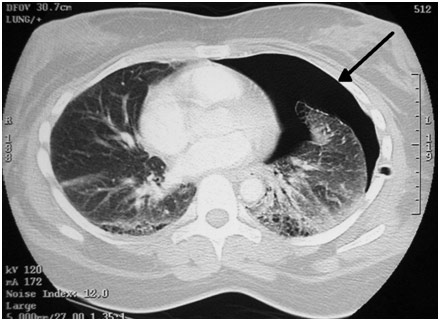
Pneumothorax
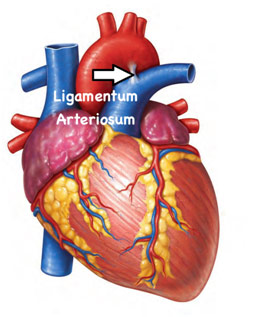
Location of the Ligamentum Arteriosum
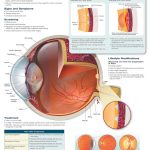
Dr. Mary Shui is an optometrist at UC Berkeley, and she spoke about the diagnostic window through the eye since oftentimes, you can see heart disease when doing a yearly eye checkup. Since there is transparent tissue in the eye and the cornea, lens, and vitreous humor are all clear, one can see the blood vessels relatively easily. In regards to identification, veins are generally darker than the arteries, and arteries are only ⅔ the width of veins.
So how does diagnostic through the eye work? For hypertension, optometrists can see that the blood vessel wall thickens due to increased pressure even though the vessel inside may be narrowed due to the atheromas (plaques). Sometimes, there can also be an aneurysm or bulging of the vessel wall, that is visible on a retinal image. For atherosclerosis in general though, alarm bells go off if the optometrist sees that the arteries are less than ⅓ the width of the veins. Additionally, optometrists are alarmed if the arteries begin to look like copper wiring, which happens because the arteries become stiff and hard, reflecting more and more light. There may also be arteriovenous nicking, which is when an artery and vein crossover, but one gets crushed because the vessel on top is stiff. This compression will cause the vessel to enlarge, causing problems like backflow and leakage. Sometimes, the vessel may also burst and cause edema in the eye, which can cause partial blindness if the blood covers the macula.
The reason why this diagnostic window is so important is that heart disease is a silent killer. Patients often do not have symptoms, so plaque can accumulate without much awareness. The plaque can then go to the heart and cause myocardial infarction or go to the brain and cause a stroke. In the eye though, you can see the plaque as the patient will have corneal arcus, a yellowish opaque ring of the eye. This Hollenhorst plaque (the name is specific to the eye) gets stuck when the eye vessel bifurcates and causes death to all of the tissue that is fed by that vessel past the blockage. Dr. Shui told us that this would be an immediate referral because, in this case, the patient has a high risk of MI and stroke as well.
Dr. Shui ended her presentation by talking about diabetic retinopathy, a dreaded complication of diabetes. The blood becomes more viscous due to platelet hyperactivity and glucose remaining in the blood. This injures the endothelial cells, which comprise the inner lining of blood vessels. This then restricts flows and causes leakages. The blood vessels will then twist to try to find a way to feed the tissue or, in proliferative diabetic retinopathy, grow new blood vessels (neovascularization) that are curly and wispy. The result is that the vessels are leakier, which can cause blindness as mentioned before, while nutrients are still not going to the tissue, which can cause necrosis. In short, the purpose of the diagnostic window of the eye is to aid in preventative medicine. It is best to stop severe complications before they even occur.
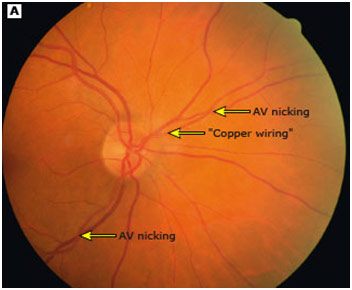
AV Nicking and Copper Wiring
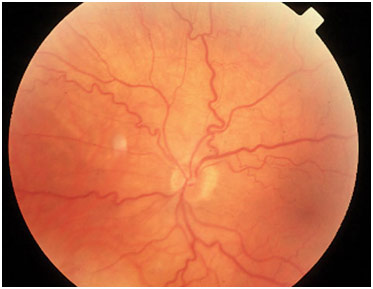
Dilated, Tortuous Retinal Veins
Going to the Cardiothoracic Summer Surgical Skills Internship at Stanford was an especially rewarding experience because I learned so much about cardiac surgery while surrounded by peers who were as excited to learn about the subject as I was. With amazing lectures and anatomy lessons combined with lab activities involving actual surgery on porcine hearts, this experience was one to never forget.
References
“Aortic Dissection.” UCSF Department of Surgery, surgery.ucsf.edu/conditions–procedures/aortic-dissection.aspx. Accessed 3 Sept. 2018.
A CT Scan of a Pneumothorax. Wikipedia, en.wikipedia.org/wiki/Pulmonary_laceration. Accessed 3 Sept. 2018.
Hypertensive Retinopathy Fundoscopy Findings. Medical Minutiae, medicalminutiae.wordpress.com/2016/11/27/retinal-pathology-part-3-hypertensive-retinopathy/. Accessed 3 Sept. 2018.
Liu, Anna. Stanford School of Medicine: Cardiothoracic Surgical Skills Summer Internship. Vimeo, vimeo.com/73051372. Accessed 3 Sept. 2018.
Maria, Faridah. Location of Ligamentum Arteriosum. 22 May 2015. Quora, www.quora.com/Is-ligamentum-arteriosum-a-closed-duct-between-the-pulmonary-artery-and-the-aorta. Accessed 3 Sept. 2018.
Olasińska-Wiśniewska, Anna, et al. “Percutaneous Balloon Aortic Valvuloplasty in Different Age Groups.” Postepy Kardiol Interwencyjnej, vol. 9, no. 1, 21 Mar. 2013, pp. 61-67, doi:10.5114/pwki.2013.34029. Accessed 3 Sept. 2018.
Pick, Adam. Side View of Mitral Valve. 2 Sept. 2008. Health Valve Surgery, www.heart-valve-surgery.com/heart-surgery-blog/2008/09/02/mitral-valve-annulus-definition-diagrams-prolapse-calcification-treatment/. Accessed 3 Sept. 2018.
Rusco, Leticia, and Joseph Sowka. Dilated, Tortuous Retinal Veins in an Impending Vein Occlusion. Review of Optometry, www.reviewofoptometry.com/article/recognizing-abnormal-vasculature. Accessed 3 Sept. 2018.
Transaortic Approach. New Heart Valve, newheartvalve.com/hcp/tavr-overview/. Accessed 3 Sept. 2018.
Will You Undergo a Surgery? Take Precautions Beforehand! 18 June 2015. In Life Group, www.inlifehealthcare.com/2015/06/18/will-you-undergo-a-surgery-take-preparations-beforehand/. Accessed 3 Sept. 2018.