From July 23rd to August 3rd, I was at Stanford for the Cardiothoracic Surgical Skills Summer Internship. It was a fascinating experience, combining surgical lab work with porcine hearts with informative anatomy lessons and lectures about cardiac surgery from various doctors. In regards to the lectures, we heard from Paul Chang about coronary artery bypass grafting surgery, Dr. Teimour Nasirov about congenital heart surgery, and Dr. Hanjay Wang about robotic and computer-assisted surgery. All were extremely informative and taught me, as well as all of the other interns, a lot about cardiac surgery, from where it began to where it will be.
Cardiac Surgery
Paul Chang, a senior scientist at Stanford who is the program director of CSSSI, gave many lectures, but one of the most interesting and informative lectures was on a type of surgery known as coronary artery bypass grafting (CABG). The surgery is usually done for patients who suffer from coronary artery disease (CAD), which is when plaque builds up in the coronary artery, which is the vessel that feeds the heart. Smoking, a sedentary lifestyle, having diabetes and eating a high cholesterol diet all contribute to CAD. Eventually, after the plaque builds up enough, the result can be a devastating heart attack where a portion of the heart dies since it cannot receive the nutrients and oxygen that are carried by the blood. Heart attacks are usually diagnosed by doing an EKG, a cardiac stress test, and looking at the heart enzyme troponin. Generally, the standard of care for heart attacks is to take the patient to the catheter lab and do a balloon angioplasty. A catheter is put into the coronary artery of the heart, using fluoroscopy and radiocontrast to ensure the catheter is in the right position. Once there, the balloon expands and contracts, deploying a stent to keep the coronary artery open. If doing a balloon angioplasty is not possible for one reason or another, that is when the cardiac surgeons are called to do a CABG.
The surgery generally begins with a median sternotomy to gain access to the thoracic cavity. After opening the pericardium, the surgeon puts the patient on cardiopulmonary bypass by putting cannulas into the aorta and right atrium with a purse-string suture around them. The venous blood comes from the right atrium of the heart, going outside the body to be oxygenated and filtered, to remove any clots, before being put back into the aorta to be pumped throughout the body. The surgeon will also cool the heart after cross-clamping the aorta so that blood does not flow through the heart. Only then does the surgeon give cardioplegia, a potassium solution designed to stop and protect the heart during the operation. It can be either retrograde (through the coronary sinus) or antegrade (through the aortic root to the coronary Ostia). The surgeon will then harvest a vein or artery from some other location in the body like the saphenous vein in the leg, the internal mammary arteries in the chest, the radial artery in the arm, the gastroepiploic artery in the stomach, or the cephalic vein in the arm. The internal mammary arteries are considered the best because they have a ten-year patency rate of 97% compared to 85% for the radial artery and 67% for the saphenous vein. Generally, the vein or artery is harvested with one end anastomosed to the aorta and the other anastomosed to just beyond the blockage in the coronary artery. However, when using the internal mammary artery, the anastomosis to the aorta is foregone as the internal mammary artery is left with its origin at the subclavian artery. Sometimes, a double, triple, or even quadruple bypass will be done based on the number of blockages present. After all the anastomosis is completed, the aorta is unclamped to allow the reperfusion of the coronary arteries, and the patient is taken off of cardiopulmonary bypass. Before closing up the chest incision, the surgeon leaves a chest tube to drain out any blood that accumulates that would have otherwise caused cardiac tamponade, which is potentially lethal. While CABG does not cure coronary artery disease, it is a particularly effective treatment that can allow a patient to live decades longer.
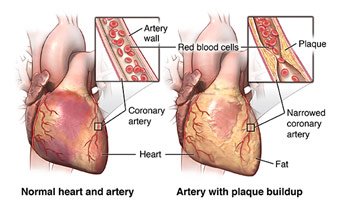
Coronary Artery Disease
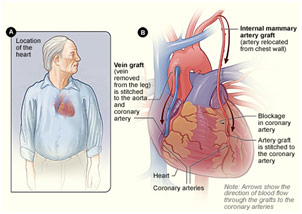
Coronary Artery Bypass Grafting
Dr. Teimour Nasirov is an assistant professor of cardiothoracic surgery at Stanford University, and he spoke about congenital cardiac surgery. The field can be summarized by the phrase “blue babies” since often time the problem is that the newborn, for one reason or another, is not getting oxygenated blood going to the rest of his body. This is clearly life-threatening and generally requires emergent surgery to correct whatever is the issue. One major congenital health conditions that he treats include hypoplastic left heart syndrome.
Hypoplastic left heart syndrome (HLHS) is characterized by an underdeveloped left side of the heart. Since the left ventricle and aorta are essential for getting oxygenated blood to the entire body, when they are underdeveloped, the right ventricle must take over the job of pumping blood to the rest of the body. The way that this happens is rather complex. Since the mitral valve is not fully developed either, oxygenated blood cannot go into the hypoplastic left ventricle and instead goes into the right atrium via an atrial septal defect (hole in the atrial wall). Through the tricuspid valve, this “purple” blood, a mix of oxygenated and deoxygenated blood, enters the right ventricle, which pumps the blood to both the lungs and the body. The blood is pumped to the body via a patent ductus arteriosus (PDA), which usually closes at birth, but stays open, or “patent,” for patients with HLHS. The PDA connects the pulmonary artery to the aorta, so the “purple” blood can go into the aorta and then throughout the body. The surgical treatment for this condition is complex and involves three separate procedures (Norwood Operation, Bidirectional Glenn Operation, and Fontan Operation).
The Norwood Operation involves first removing the atrial wall (atrial septectomy) so that oxygenated blood can freely go from the left atrium to the right atrium (and right ventricle) before ligating the PDA to prevent any blood from flowing through it. They open the aortic arch, the pulmonary trunk, and the descending aorta before suturing them together along with a graft in order to create a “neo-aorta” that is stronger and can handle high-pressure blood flow. A Blalock-Taussig shunt is then placed to connect the innominate artery, which branches from the aorta), to the pulmonary artery so that blood can still get to the lungs. If a Sano modification is done, the shunt will be placed to connect the right ventricle of the heart to the pulmonary artery. Thus, this procedure does not solve the “purple” blood issue but allows the infant to not have to take prostaglandins to keep the PDA open and creates a new way for blood to flow throughout the body because the right ventricle is now pumping blood to it it.
The Bidirectional Glenn Operation involves first ligating the Blalock-Taussig shunt that had been placed in the Norwood Operation. Then the superior vena cava is transected and connected to the pulmonary artery in the same place where the Blalock-Taussig shunt was connected. Therefore, deoxygenated blood from the upper body can go directly into the pulmonary artery and lungs in order to get oxygenated. This helps to reduce the mixing of deoxygenated and oxygenated blood in the right atrium and right ventricle.
The Fontan Operation is the final stage operation, and it begins by transecting the inferior vena cava. The inferior vena cava is then connected to an extracardiac Fontan (graft), which is then connected to the undersurface of the pulmonary artery. The surgeon can also do a lateral tunnel Fontan where the surgeon connects the inferior vena cava to the inferior part of the superior vena cava by created a tunnel (baffle) within the right atrium. That end of the superior vena cava is then also connected to the right pulmonary artery.
Sometimes, a fenestration (hole) may be made in that graft and the right atrium in order to create another shunt that helps to reduce the risk of pleural effusion as the Fontan circuit pressure can “pop-off” into the heart. It is also worth noting that the blood is only able to make it to the lungs without being pumped by the heart because as the baby becomes older, the pulmonary resistance decreases, so passive blood flow is enough to get the blood to the lungs and back.
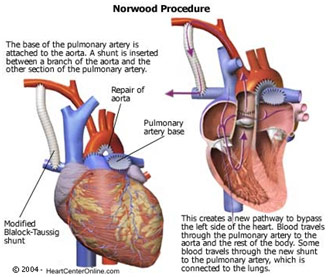
Norwood Operation
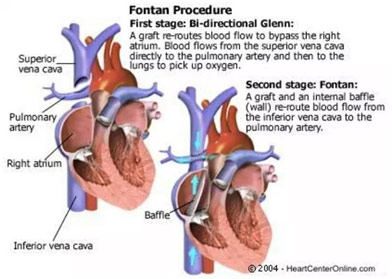
Bidirectional Glenn Operation, and Fontan Operation
Dr. Hanjay Wang is a cardiothoracic resident at Stanford, currently doing a research fellowship, and he spoke on robotic and computer-assisted surgery. He began by talking about the history of robotic surgery with phrases like “to cut is to cure” and “the greater the surgeon, the bigger the incision.” The problem with those kinds of statements is that they undermine the fact that larger incisions have poorer aesthetic outcomes and get infected more easily. Surgeons have realized this and have started to do more and more surgeries laparoscopically through tiny keyhole incisions, which are only there to insert the tools. There are many disadvantages of laparoscopic surgery like 2D camera visualization that does not allow for anatomical depth, counterintuitive motion planning where the surgeon’s and the camera’s position makes moving the instrument correctly difficult, restricted degrees of motion since the instruments are more rigid than the surgeon’s hand, and increased tremors because the instrument is far out from where the surgeon is controlling it. Thus, in the 1980s, people began to believe that machines could help solve some of these problems. There was the PUMA 560 in 1985, ROBODOC of 1992, ZEUS robotic surgical system of 1998, and the Da Vinci Surgical System of 2000, which has now become the preeminent robot that surgeons use in the operating room. To give a brief summary of how it works, a surgeon sits at a counsel while an assistant loads tools on robotic arms that hang over the patient and do the physical motions of the operation.
Like many things in our world, there are both advantages and disadvantages of the Da Vinci Surgical System. First, the surgeon gets a crisp high-resolution 3D rendering of the view (up to 10 times magnification), allowing proper identification of anatomical structures in their natural colors. Additionally, the instruments have a much greater range of motion since the human hand has four degrees of freedom while the robots have seven degrees of freedom. Hand tremors are minimized because surgeons themselves are not operating and the system can ignore stimuli that it perceives to be a surgeon having a hand tremor at the console. On top of that, the da Vinci Surgical System can do surgeries through a very small incision, which, as mentioned before, is good for cosmetics and infection control. Lastly, surgery can be done from far away: in the Lindbergh Operation, a surgeon in New York did a laparoscopic cholecystectomy on a patient in France with only milliseconds of lag time. The possibilities are endless since surgeries can be done in third world countries and battlefields as well while the surgeon is back home.
However, the possibilities are also restricted for several reasons but mostly time, money, and lack of better outcomes. To start off, it takes a long time to learn how to do robotic surgery, and most surgeons need to dedicate an entire year after they finish their training to be adept at it. Additionally, the entire team needs to be able to do this since there are different nursing, anesthesia, and instrument requirements for robotic surgery. These surgeries also just take longer to do, which means fewer patients are taken care of and less money is made for the hospital. Furthermore, money is an issue since it takes two million dollars to buy a robot and hundreds of thousands of dollars to maintain it every year. Surgeries across the board are thus more expensive if done robotically, and there is always that opportunity cost of the additional time spent doing robotic surgeries. Lastly, in general, there is no reduction in complication rates, hospital stay lengths, nor operating times when using robotic surgeries. While these disadvantages are slightly harrowing, Dr. Wang assured us that surgical technology will get better and more advanced, especially as Intuitive Surgical loses market share as a result of more competition.
Dr. Wang also gave us an insight into the future of surgical technology. He first brought up incisionless surgery where miniature robots are swallowed or inserted through narrow orifices and can be wirelessly controlled by the surgeons. There is already some incisionless surgery being done. Dr. Wang brought up an example where surgeons insert an endoscope into the esophagus, guide it to the stomach, and then poke a hole to enter the abdominal cavity. They then remove the gallbladder and take it out of the patient’s mouth. Dr. Wang also brought up computer-assisted surgery and automated robotic surgery. The former would allow the planning out of surgery to getting the exact anatomy of the patient. If combined with 3D printing, surgeons could practice the steps of a difficult surgery before they actually have to do it. The latter regards making the robot do a surgery themselves. While this seems especially promising, the foreseeable future indicates that society is nowhere close to developing it nor dealing with the liability issues that will come associated with it.
To end his talk on robotic and computer-assisted surgery, Dr. Wang talked about the far future where scientists would redesign existing cells or use new cells to do “surgery.” These autonomous synthetically bioengineered cellular robots would be easy to deliver, totally non-invasive, have a cellular level precision, and would not waste resources like surgery does today. Already, there are some bacteria designed to destroy plaque in arteries or target cancer cells, but in the far future may have surgery be entirely replaced with these cellular robots.
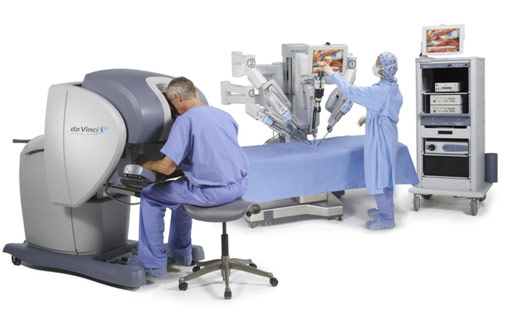
Setup of the da Vinci Surgical System
Going to the Cardiothoracic Summer Surgical Skills Internship at Stanford was an especially rewarding experience because I learned so much about cardiac surgery while surrounded by peers who were as excited to learn about the subject as I was. With amazing lectures and anatomy lessons combined with lab activities involving actual surgery on porcine hearts, this experience was one to never forget.
References
Coronary Artery Bypass Grafting. UCSF Cardiac Surgery, cardiacsurgery.ucsf.edu/conditions–procedures/coronary-artery-bypass-grafting-(cabg).aspx. Accessed 4 Sept. 2018.
da Vinci Surgical System. Standard Journal, www.rexburgstandardjournal.com/news/local/madison-memorial-to-implement-robotic-surgery/article_d3b6e402-9a0c-11e6-bab4-472f05e6e632.html. Accessed 4 Sept. 2018.
Fontan Procedure. Pinterest, i.pinimg.com/originals/77/dd/4b/77dd4b7339ed81d5ae616e499d814a07.jpg. Accessed 4 Sept. 2018.
“Hypoplastic Left Heart Syndrome (HLHS).” Cincinnati Children’s Hospital, www.cincinnatichildrens.org/health/h/hlhs. Accessed 4 Sept. 2018.
“Information for Patients and Parents about the Fontan Operation.” The Royal Children’s Hospital Melbourne, www.rch.org.au/cardiology/parent_info/Information_for_patients_and_parents_about_the_Fontan_Operation/. Accessed 4 Sept. 2018.
“Medical Animation: Glenn Operation – Cincinnati Children’s.” Youtube, www.youtube.com/watch?v=kBh8YDqcqxc.
Normal Heart and Artery Vs. Artery with Plaque Building. Johns Hopkins Medicine, www.hopkinsmedicine.org/healthlibrary/test_procedures/cardiovascular/coronary_artery_bypass_graft_surgery_92,P07967. Accessed 4 Sept. 2018.
Norwood Operation. Severin Grant Brenny, www.severinbrenny.com/norwood_operation.html. Accessed 4 Sept. 2018.
Will You Undergo a Surgery? Take Precautions Beforehand! 18 June 2015. In Life Group, www.inlifehealthcare.com/2015/06/18/will-you-undergo-a-surgery-take-preparations-beforehand/. Accessed 3 Sept. 2018.