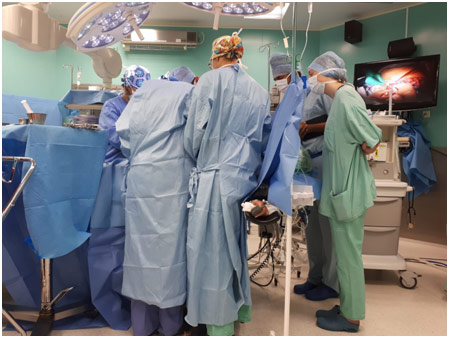
For 2 weeks in August 2018, I was in Warsaw, Poland for the Gap Medics Internship. The experience was fascinating, allowing me to observe surgeries from the same vantage point as the operating doctor while also giving me the opportunity to learn so much more about medicine. My first week in Poland was spent in the gastroenterology and transplantation department while my second week was spent in the orthopedics and traumatology department. Both weeks proved especially insightful and just showed how life-changing, and hard, medicine truly is. In this post, I will be discussing some gastrointestinal surgeries like a simultaneous pancreas and kidney transplant, a jejunostomy, and an abdominoperineal resection with a right hemicolectomy.
Gastrointestinal Anatomy
A simultaneous pancreas and kidney transplant (SPK) is one of the rarest and most technically challenging types of surgeries there is with fewer than 1000 done in the US each year. I was, fortunately, able to see this operation on my first night, coming into the hospital at 1 AM and staying until the surgery’s terminus at 5 AM. An SPK is used to cure a patient with Type 1 Diabetes as the pancreas is responsible for the creation of insulin, and in Type 1 Diabetes, the patient’s own pancreas is not making sufficient, or any, insulin. An SPK is the most common type of pancreas transplant; however, a pancreas transplant alone (PTA) and pancreas after kidney transplant (PAK) can also be done. As this procedure is very invasive and carries the risk of many complications, SPK is generally only offered to those with life-threatening complications from T1D (like hypoglycemic unawareness) and with severe kidney disease (like an end-stage renal disease). With a new pancreas and kidney, the patient can now produce their own insulin and manage damage to other structures like their eyes or nervous system, but they have to take immunosuppressants for the rest of their life to prevent immune attacks to their new pancreas and kidney.
After being transported from the brain-dead donor, the kidney was in ice when I walked into the OR as the surgeons sat around it, preparing the kidney by ligating various vessels and making sure that the renal artery, renal vein, and ureter were ready for their anastomosis to the recipient’s vessels. For the pancreas, which would be transplanted along with a section of the duodenum, the surgeons were constructing the portal vein, the superior mesenteric artery, the superior mesenteric vein, the splenic artery, and the splenic vein. The surgeon connected the donor’s splenic artery and the donor’s superior mesenteric artery to a Y graft made of the donor’s common iliac artery bifurcation into the internal iliac artery and the external iliac artery.
After preparing both organs, the kidney was the first to be transplanted after the surgeons created a pocket for it to lie in the iliac fossa. The kidney receives oxygenated blood via the renal artery, gets rid of deoxygenated blood via the renal vein, and disposes of waste products via the ureter. All three of these need to be reattached to the recipient’s vascular and urinary system. The recipient’s external iliac vein was clamped and cut before the donor’s renal vein was anastomosed to the external iliac vein in an end-to-side fashion. Following the kidney’s venous attachment, the surgeon created another opening in the recipient’s external iliac artery before “parachuting” the donor’s renal artery to it in a similar end-to-side anastomosis. He then went on to anastomose the ureter to the bladder, making sure the connection was tight. Soon after, the kidney became a light pink as it was reperfused with oxygenated blood.
The pancreas was then transplanted with the splenic artery and superior mesenteric artery being connected from the donor’s pancreas to the recipient’s external iliac artery via the Y graft described earlier. This gave the pancreas its arterial blood supply. Afterward, the superior mesenteric vein and the splenic vein were connected to the recipient’s common iliac vein via the donor’s portal vein. This reconstructed the pancreas’ venous blood supply. The donor’s duodenum attached to the pancreas was also anastomosed to the recipient’s duodenum in a side-to-side fashion so that the exocrine material (digestive enzymes) from the pancreas could drain through the pancreatic ducts into the donor’s duodenum and, from there, into the recipient’s duodenum.
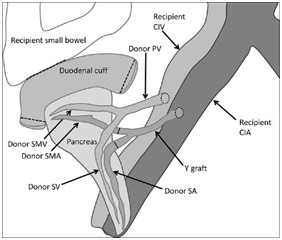
Donor Pancreas Anastomosis to Recipient
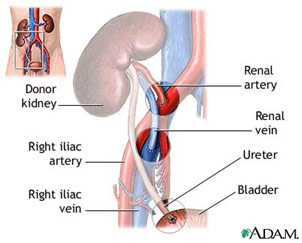
Donor Kidney Anastomosis to Recipient
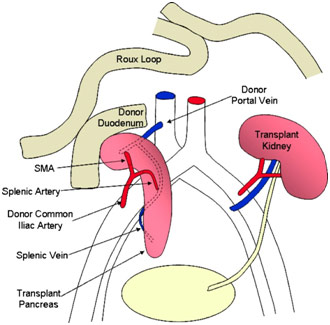
Anatomy After Simultaneous Pancreas and Kidney Transplant
One of the surgeries I saw was of a patient with gastroparesis, paralysis of the stomach muscles; the patient could not empty her stomach easily. A few months ago, she was involved in a motor vehicle accident that led to a traumatic brain injury and subsequent neurosurgery. Part of the result of the MVA was damage to the nervous system and, more specifically, the vagus nerve. Gastroparesis while causing symptoms like heartburn or bloating can also cause life-threatening infections or solid bezoars that block the stomach entirely. The main issue, however, is feeding the patient. The patient can be given parenteral nutrition (through the veins) or enteral nutrition (through the GI tract); the latter is preferred. Either way though, surgery is needed on an emergent basis.
After the neurosurgery, the patient had a gastrostomy tube fed into the stomach as this was before the gastroparesis was known about. The gastrostomy tube was clearly ineffective, so I observed the surgeon perform a jejunostomy, the placement of a feeding tube into the patient’s jejunum, the second part of the small intestine. The surgeon opened the patient up and was searching for the ligament of Treitz as that is a landmark for the insertion point in the jejunum. In that pursuit, he was lifting the omentum and small intestines out of the body. Once the appropriate location was found, the surgeon used a blue rubber material to mark that loop of the bowel as a precautionary measure so that if he dropped the bowel, it would be easy to find the correct spot again. Then, the surgeon made a 4 cm opening in the jejunum and inserted a cannula to help guide the feeding tube in. Once the tube was inserted, the cannula just peeled off, and the surgeon used a purse-string suture to close the incision in the jejunum so the tubing would remain firmly in place. The tubing was then guided out of a small hole he made in the skin with fixation sutures keeping the J-tube in place.
The tubing was special as it was filled with holes to allow for effective nutrition. Additionally, it was not put in the duodenum as the duodenum is too close to the paralyzed stomach and risked being ineffective. Additionally, it is significantly more difficult to access the duodenum since the duodenum is located deep in the abdomen and is difficult to bring up to the skin, unlike the jejunum. However, the tube is not put in the ileum, the third part of the small intestine, either because a great deal of nutrient absorption happens in the small intestine, and the patient should have the food moving through the small intestine for as long as possible.
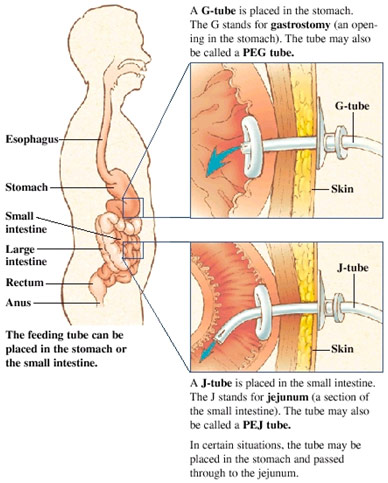
G-tube vs. J-tube insertion sites
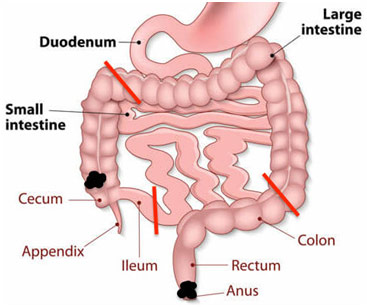
One of the most complex and invasive procedures I saw were synchronous tumors in the anus and right colon, meaning there were two separate primary tumors found at the same time in two separate regions. The surgery is done, an abdominoperineal resection with a right hemicolectomy, removed much of the abdominal contents. More specifically, the abdominoperineal resection included the excision of the anus, rectum, and sigmoid colon while the right hemicolectomy included the excision of the ascending colon.
There were two openings through which the surgery was carried out. The right hemicolectomy was done first and was done entirely through the abdominal incision while the abdominoperineal resection was done primarily through the anus. The right hemicolectomy saw the surgeon access the abdominal cavity via a midline incision and then mobilize the large intestine, or colon, after retroperitoneal dissection to move the greater omentum of the transverse colon and remove the colon adhesions to the posterior abdominal wall. The surgeon moved on to ligate the right colic and right ileocolic arteries that feed the ascending colon. The large intestine was divided at a specific point of the transverse colon with a stapler, and the ileum and its mesentery are cut to externalize the ascending colon in its entirety. The remaining ileum was then anastomosed to the transverse colon with an end-to-end anastomosis to preserve the integrity of the GI tract.
After the removal of the ascending colon, the abdominoperineal resection began. Some more dissection was done to gain access to the posterior abdomen and remove the adhesions to the sigmoid colon and the rectum. The blood supply through the superior hemorrhoidal vessels is ligated before the sigmoid colon was stapled to remove its attachment to the descending colon. At that moment, the surgeon transitioned to the perineal position and continued the surgery from the patient’s anus: the patient was in the lithotomy position with their knees up and hips abducted. Following an elliptical incision of the skin, the muscles and other attachments to the anus are cut, and the inferior hemorrhoidal vessels are ligated. Finally, the anus, rectum, and sigmoid colon were removed in the perineal position. The elliptical incision was then closed. Because the rectum and anus are out, there needed to be some way for the excretory material to get out. Thus, a small circular incision was made in the skin of the patient’s abdomen and the remaining colon end was brought out to the surface of the skin. With a ring to hold the intestine in place, the intestine was sutured in place. This is known as a stoma and requires a colostomy bag to capture the patient’s stool. An abdominal tube was left to drain any remaining blood, and the midline incision was sutured close.
Tumor Locations (Black) and Incision Locations (Red)
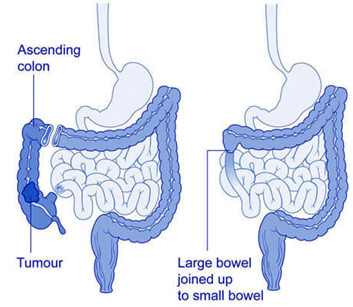
Anatomy Before and After Right Hemicolectomy
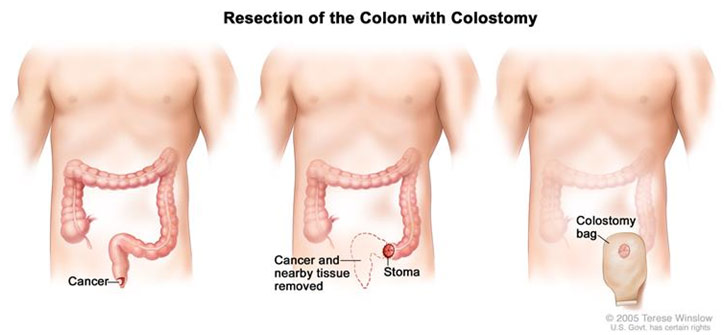
Anatomy Before and After Abdominoperineal Resection
All in all, the Gap Medics experience was one of a kind. I got such a wonderful opportunity to watch surgeries from an unbelievably close distance thanks to amazing mentors like Dr. Agnieszka. I saw surgeries done nearly flawlessly as well as surgeries with a few inadvertent complications, helping me to get a realistic picture of what medicine looks like. The experience was both rewarding and unforgettable.
References
Abdominoperineal Resection. NIH, www.cancer.gov/publications/dictionaries/cancer-terms/def/abdominoperineal-resection. Accessed 25 Nov. 2018.
Anastomoses for Pancreatic Transplant. NCBI, www.ncbi.nlm.nih.gov/pmc/articles/PMC4247503/. Accessed 25 Nov. 2018.
The Digestive System. Scientific Publishing Ltd., www.scientificpublishing.com/product/the-digestive-system/. Accessed 25 Nov. 2018.
G-Tube & J-Tube (PEG). Pinterest, www.pinterest.com/pin/362610207467760561/?lp=true. Accessed 25 Nov. 2018.
Heller, Matthew, and Puneet Bhargava. “Imaging in Pancreatic Transplants.” NCBI, 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC4247503/. Accessed 25 Nov. 2018.
The Large Intestine. Kids Biology, kidsbiology.com/human-biology/the-large-intestine/. Accessed 25 Nov. 2018.
“Open Right Hemicolectomy.” Surgical Operations, 30 Sept. 2007, proceduralist.blogspot.com/2007/09/open-right-hemicolectomy.html. Accessed 25 Nov. 2018.
Perry, W. Brian, and J. Christopher Connaughton. “Abdominoperineal Resection: How Is It Done and What Are the Results?” Edited by Harry L. Reynolds. NCBI, Aug. 2007, www.ncbi.nlm.nih.gov/pmc/articles/PMC2789508/. Accessed 25 Nov. 2018.
Preop Check. Med UOttowa, wiki.med.uottawa.ca/display/WIKITHESIA/Renal+Transplantation. Accessed 25 Nov. 2018.
Right Hemicolectomy. Cancer Society, cancernz.org.nz/cancer-information/cancer-types/bowel-cancer-and-bowel-function/surgery/. Accessed 25 Nov. 2018.
Schematic Diagram of SPK Transplant, with Enteric Drainage. NCBI, www.ncbi.nlm.nih.gov/pmc/articles/PMC3259388/. Accessed 25 Nov. 2018.
Tapia, J., et al. “Jejunostomy: Techniques, Indications, and Complications.” NCBI, 23 June 1999, www.ncbi.nlm.nih.gov/pubmed/10227930. Accessed 25 Nov. 2018.