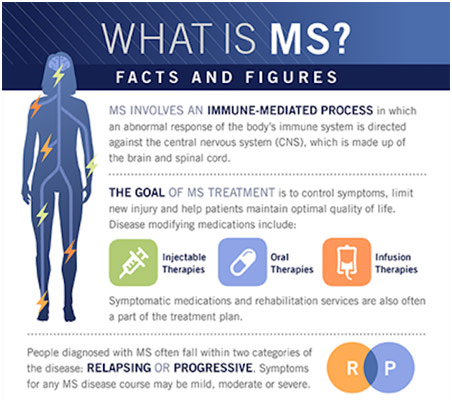
A few months back, I took a Johns Hopkins CME Course on a neurological condition known as multiple sclerosis (MS). MS is a rare autoimmune disorder where the patient’s immune system attacks its own self, specifically the central nervous system of the brain and spinal cord. The cause of MS is unclear, but some genetic and environmental factors are thought to be related to the disorder. For example, some risk factors include being a woman, who have 2-3 times higher chance of getting MS than men, and living in areas of higher latitude where there is less vitamin D. Early diagnosis is especially important because nerve damage and brain atrophy can be reduced if treatment is started earlier. However, the nonspecific symptoms of MS make the diagnosis hard. Thus, awareness about the disorder (its pathophysiology, its symptoms, its diagnosis, and its treatment) are vital to ensuring the best possible result for patients with MS.
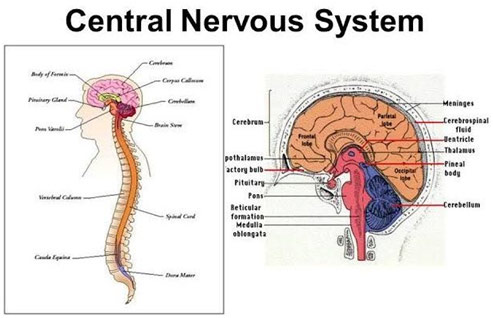
Central Nervous System
Multiple sclerosis sees the demyelination of neurons of the brain and spinal cord. Myelin is a fatty layer around the axon of the neuron that helps increase the speed of signal transmission by limiting the dissipation of the electrical charge. The improper destruction of myelin by T-cells, which get past the blood-brain barrier despite normally not being able to do so, destroys the communication of the nervous system. These T-cells then release cytokines, proteins vital to cell signaling, which leads to the breakdown of the blood-brain barrier. The resulting increased permeability allows more T-cells to get in as well as B-cells and macrophages. The B-cells mark the myelin for destruction while the macrophages then also destroy the myelin. This causes inflammation and leaves certain lesions (T2 or Gd-enhancing) in the white matter of the brain. However, in RRMS (relapse remitting multiple sclerosis), the most common type of MS, this whole process happens in periodic spells as there is the remyelination of the nerves with the help of oligodendrocytes, allowing for some healing. This is never complete though, and the disability generally only gets worse and worse.
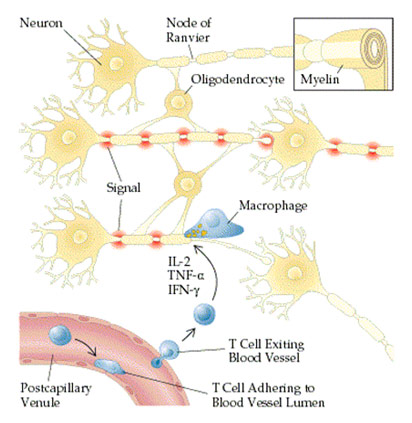
T-cells Entering Blood-Brain Barrier and Causing Cytokine Release Following Demyelination
Symptoms of multiple sclerosis are vastly varied based on the location of the lesion formation. However, common symptoms include dysarthria (speech problems) due to plaques in the brainstem; nystagmus (uncontrolled ocular movements) and impaired vision due to plaques in the ocular nerves; tremors and spasticity (muscle stiffness) due to plaques along the motor pathway of the spinal cord; numbness and paresthesia (tingling) due to plaque along the sensory pathway of the spinal cord; poor judgment and memory due to plaques in the frontal lobe of the brain; and fatigue. There is no direct test for MS; however, an MRI, spinal tap, and evoked potential test can help rule out other disorders and ascertain that MS is the one at play. An MRI looks for brain lesions and plaques as they look like small white specks. A spinal tap can look for an elevated antibody count in the cerebrospinal fluid, indicative of an autoimmune disorder. An evoked potential test that measures the brain’s electrical activity when the patients receive a sensory stimulus can help see if there has been demyelination since then the electrical signal will transfer slower than normal.
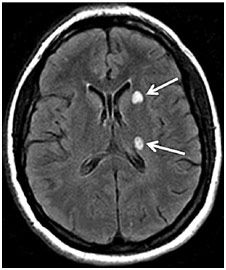
Brain Lesions on MRI in Patient with MS
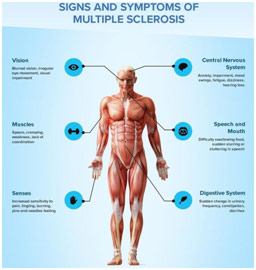
Common Symptoms of MS
Medications for multiple sclerosis, specifically RRMS, seek to reduce the frequency of relapses and reduce the severity of relapses. Clinicians seek that their patients achieve NEDA-3 criteria where there are no relapses, no evidence of disease activity on MRI, and no progression of the disability. To achieve this end, they use injectable, oral, and infusible drugs. The two most significant first-line, injectable drugs are interferon beta and glatiramer acetate, and they are generally used to treat milder cases of multiple sclerosis. Both work by switching from the Th1 to Th2 profile, from the proinflammatory to the anti-inflammatory profile. Additionally, they both suppress the activity of T cells and B cells that degrade the myelin while increasing the activity of regulatory T cells that protect the myelin. Clinical trials have shown that interferon beta has reduced relapse rate by about 30%, reduced Gd-enhancing and T2 lesions by 50-80%, and reduced disability progression by 30%. Glatiramer acetate has similar effects. While interferon beta can cause many adverse effects like depression and headaches, glatiramer acetate is generally very safe, technically not requiring any blood work.
The three major oral medications, which are a higher tier to the injectable drugs, are fingolimod, dimethyl fumarate, and teriflunomide. Fingolimod is an S1P receptor modulator, working by preventing lymphocytes like T and B cells from moving around and finding the antigen to trigger the demyelination. While effective, a great deal of screening is needed as the S1P receptor is found on, not only the lymphocytes, but also cardiac myocytes, the retina, and the liver. The drug has the potential to cause AV block in the heart (conduction impaired causing arrhythmia), macular edema in the eye (fluid buildup causing vision issues), and liver injury. However, after this initial monitoring for any of these potential side effects, this medication is well tolerated aside from the potential for infections in the long term that come from the modulation of the immune system. Dimethyl fumarate is another oral medication that, like the injectable drugs, shifts from the proinflammatory Th1 to the anti-inflammatory Th2 response and suppresses the lymphocytes. The unique aspect of this drug is that it activates the neuroprotective Nrf2 pathway. It has the same 50% reduction in relapse rates as Fingolimod as well as an 80% reduction of Gd-enhancing lesions and a 30% reduction in disability production. While GI adverse effects, as well as flushing, can occur, they can generally be managed with steps like slowly bringing up the dose of the medication and taking a baby aspirin daily. The most serious concern is the potential for lymphopenia, or low lymphocyte count, which leads to a higher risk of infection, a potential reason to discontinue this treatment. The last oral medication is teriflunomide, which works by inhibiting the enzyme dihydroorotate dehydrogenase, which is integral to lymphocytes. This basically suppresses T and B cells. However, the clinical effectiveness is limited compared to the other two oral medications, and the medication is teratogenic to a fetus, effectively barring its use from women trying to get pregnant.
The four major infusible medications, a higher tier than oral drugs, are natalizumab, alemtuzumab, ocrelizumab, and daclizumab. Natalizumab is given by IV infusion every 4 weeks, and is an alpha 4 beta 1 integrin inhibitor to, like fingolimod, limit the movement of the lymphocytes so that they are not able to get to the CNS. This drug highly suppresses disease activity with the AFFIRM trial showing a 68% reduction of relapse and a 92% reduction of Gd-enhancing lesions. While there may be some reactions early on, the drug is also well-tolerated in the long run. The only issue is that the patient has to be JCV seronegative, or not have any of the John Cunningham viruses, even in its dormant state. In JCV seropositive patients, there is a significant risk of using natalizumab. Alemtuzumab, an anti-CD52 monoclonal antibody, is a type of induction therapy where it is given intravenously for 5 days in a row and has some long term effects. It works by causing the apoptosis of lymphocytes in the blood and bone marrow through binding to the CD52 antigen on both normal and abnormal lymphocytes. This “resets” the immune system and is effective in managing the disorder in the long run, despite the absence of continuous treatment, for some patients. However, autoimmune complications must be monitored monthly for four years as that is a serious side effect. Ocrelizumab, an anti-CD20 monoclonal antibody, is also a highly efficacious therapy with 46% reduction in relapse rate and 94% reduction in Gd-enhancing lesions when compared to interferon beta, not a placebo. While long-term effects are not well understood, it is known that B-cell depleting therapies (this drug induces B cell apoptosis through the CD20 antigen) can cause a patient to be suspect to a variety of infectious diseases. Thus, screening for hepatitis, tuberculosis, varicella, and HIV are done among other screens. Lastly, daclizumab, an anti-CD25 monoclonal antibody, blocks the IL-2 receptor of T-cells to reduce T-cell proliferation.
Switching the therapy is only done if the MRIs and the patient’s symptoms are indicative of relapse. For instance, if there are two or more T2 lesions or 1 or more Gd-enhancing lesions, then relapse is likely. In the end, the physician is targeting the NEDA-3 criteria, so if a drug is not achieving that, you may have a breakthrough disease, which is when a disease does not respond to treatment. Using evidence-based medicine, one may change to therapy of a different mechanism or action and/or escalate the therapy from injectable to oral/infusible for example.
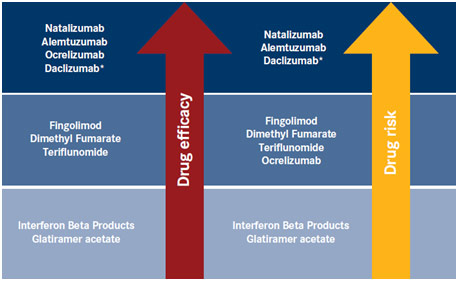
Relative Efficacy and Risk of FDA-Approved Medications for Multiple Sclerosis
Multiple Sclerosis has no cure, but with proper care, it is entirely possible to live a healthy life free of worry from serious complications. With more awareness, more people can understand this complex disease and be able to express sympathy to those who suffer from its mostly silent effects. Additionally, proper management and treatment, especially if begun early, can go a long way in improving one’s quality of life.
References
Central Nervous System. Infectious Diseases Congress, infectiousdiseasescongress.alliedacademies.com/2018/events-list/central-nervous-system-infections. Accessed 3 Jan. 2019.
Marvanova, Marketa. MRI Image of 38-year-old Woman with Multiple Sclerosis. Research Gate, www.researchgate.net/figure/MRI-Image-of-38-year-old-woman-with-multiple-sclerosis-The-arrow-points-toward-two-brain_fig6_305681128. Accessed 3 Jan. 2019.
“Multiple Sclerosis – Causes, Symptoms, Diagnosis, Treatment, Pathology.” Youtube, uploaded by Osmosis, 22 Mar. 2017, www.youtube.com/watch?v=yzH8ul5PSZ8. Accessed 3 Jan. 2019.
“Multiple Sclerosis FAQs.” National Multiple Sclerosis Society, www.nationalmssociety.org/What-is-MS/MS-FAQ-s. Accessed 3 Jan. 2019.
“Multiple Sclerosis in the Age of Enhanced Therapeutic Options.” MS-Leaders, uploaded by Johns Hopkins Medical School, www.ms-leaders.org/Workshop/MSLeadersWorkshop/Pages/Default.aspx?WorkShopId=18&CmeType=jhu&UserKey=I3aXhLuLDmM0JG1AClgxBaXWf3_fN_t_&skiplanding=true. Accessed 3 Jan. 2019.
“Multiple Sclerosis Signs and Symptoms – Nervous System Diseases – NCLEX-RN – Khan Academy.” Youtube, uploaded by Khanacademymedicine, 23 June 2015, www.youtube.com/watch?v=A8ZK4VWmbGc. Accessed 3 Jan. 2019.
Relative Efficacy and Risk of FDA-Approved DMT. AJMC, www.ajmc.com/journals/supplement/2018/multiple-sclerosis-review-of-diagnosis-and-management/ms-the-safety-efficacy-balance-and-preventing-neurodegeneration. Accessed 3 Jan. 2019.
Signs and Symptoms of Multiple Sclerosis. Find a Top Doc, www.findatopdoc.com/Healthy-Living/What-Are-the-Signs-and-Symptoms-of-Multiple-Sclerosis. Accessed 3 Jan. 2019.
Structure of Neuron and Mechanism of Demyelination. Multiple Sclerosis, www.mult-sclerosis.org/news/Jan2003/FullTextDemyelinatingDiseases.html. Accessed 3 Jan. 2019.
What Is MS? Penn Medicine, www.pennmedicine.org/for-patients-and-visitors/find-a-program-or-service/neurology/multiple-sclerosis/treating-ms. Accessed 3 Jan. 2019.