In late May 2020, I, along with over 600 other high school students from around the world, virtually attended the Global Health and Leadership Conference hosted by Harvard College VISION. The conference featured an impressive array of workshops, competitions, and speakers with the mission to inspire students about global health and empower them to become leaders shaping policy for the greater good. From Refugee & Migrant Health to Leadership in Federal Government, the conference was themed around the idea of health in an increasingly connected world, perhaps never more relevant than during the COVID-19 pandemic. In this first part of a three-part series, I want to focus on one of the speakers who presented at this conference, specifically Dr. Norman Sharpless, Director of the National Cancer Institute (NCI). The NCI is one of the world’s largest funders of cancer research with $6 billion allocated to them by Congress. In fact, the NCI is the largest and oldest of the National Institutes of Health and is tasked with developing foundational sciences that lead to therapeutics, diagnostic tools, etc. for cancer.
After completing a clinical and research oncology fellowship at Boston’s Dana-Farber Cancer Institute, Dr. Sharpless ran a basic sciences lab at UNC-Chapel Hill, studying cancer in genetically engineered mice and eventually becoming the Director of the UNC Lineberger Comprehensive Cancer Center. Dr. Sharpless characterized his career in academia as extremely rewarding while also acknowledging the intense pressure to publish results, get funding, and train students/residents. Federal work, on the other hand, has Dr. Sharpless working an almost entirely administrative role where he, among other things, manage the funding of the NCI and ensures that the gamut of oncology research is rational and balanced (eg. not all jumping to one approach such as angiogenesis inhibition). Dr. Sharpless encouraged all of us to consider a career in public health and health policy because the work is truly fulfilling and the problems you are asked to solve are truly exhilarating.
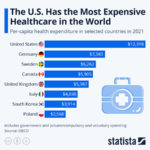
For Dr. Sharpless, one of those problems includes dealing with the COVID-19 pandemic. While this may be unusual for the NCI, it is not unheard of: during the HIV/AIDS epidemic in the 1980s, the NCI devised the first successful therapeutics for the disease (azidothymidine, didanosine, and zalcitabine), transforming HIV/AIDS from a death sentence to something that could be managed. As a result, the NCI has played a crucial role in fighting the COVID-19 pandemic, tapping their network of some of the best scientists in the nation whose specialty in virology and the immune system proves invaluable. Additionally, the NCI has been leveraging its existing serology testing for HPV and retooling them into serology testing for COVID-19. More popularly known as antibody tests, serology tests are critical to be able to identify those who have been infected by the virus and are immune, an important task as the nation moves towards reopening. In fact, Congress has recognized and supported the NCI’s role in serology testing, allocating $306 million in emergency funding for this purpose.
Serological testing is also a great example of how the NCI works closely with other federal agencies interested in public health, such as the Food and Drug Administration (FDA). The FDA typically has to be very vigilant of conflict of interest concerns because approving a healthcare product always means creating a winner and loser: the company whose product was approved and that company’s competition, respectively, leading to millions of dollars of profits and losses. Thus, having a bias in the review process can be extremely deleterious. This concern is largely why the FDA cannot simply go out and ask experts in academia for their assistance, instead of seeking out the NCI as a trusted, neutral source to provide scientific advice. In the current pandemic, the FDA has been overwhelmed with the over 200 manufacturers that have sent them serological testing for approval. As a result, the FDA gave these devices to the NCI, which has ever since been providing results back to the FDA to help with regulation.
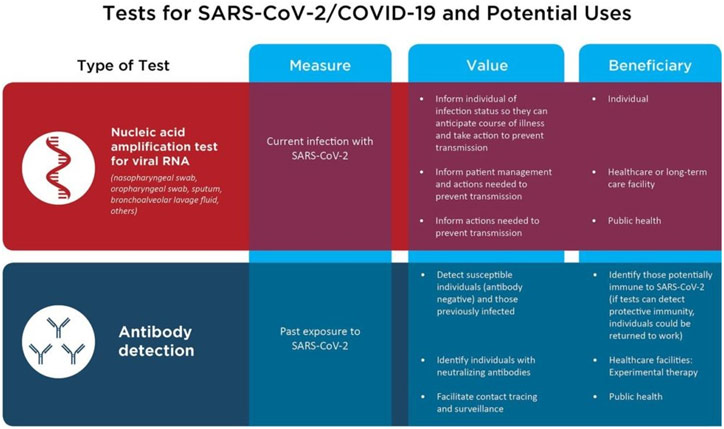
Two Types of Testing for COVID-19
Dr. Sharpless also shared that he is worried about COVID-19’s impact on cancer outcomes. With cancer patients scared of COVID-19 as their weakened immune systems put them at increased risk of contracting the disease, they are often delaying or deferring care, from not going for a mammogram to not getting thoracic surgery to remove potentially malignant nodules. Interestingly, cancer drugs, which are perpetually in shortage because of complex international supply chains (eg. every drug used to treat pediatric leukemia has been in a shortage at some point), are not currently in shortage in the U.S. However, the reason behind this availability underscores an uncomfortable reality: patients are not coming to the hospital to get treatment. Dr. Sharpless is concerned that cancer outcomes will become significantly worse in the coming years as cancers grow unchecked now.
These diagnosis and treatment delays are compounded with delays in research as well. For example, the NCI was going to fund a new initiative to encourage international teams of cancer researchers, working with the Cancer Research UK (CRUK), but these plans were delayed because of COVID-19. The biggest disruption to cancer research, however, relates to clinical trials. Before the COVID-19 pandemic, the FDA was approving cancer drugs at a record pace (20-30 a year), and these drugs were incredibly effective. As patients stay home because of COVID-19, clinical trial accrual has dropped by about half, which means that clinical trials will take about twice as long to complete, and, as a result, the development/approval of new drugs will suffer. The NCI has been doing what it can, encouraging researchers to write manuscripts, being flexible so that scientists do not have to lay off staff, and incentivizing telehealth to allow clinical trials to proceed, but cancer research will, unfortunately, suffer nonetheless.
Delay in Vital Cancer Care an Uncomfortable Reality
Towards the end of the session, I asked Dr. Sharpless the following question: how do you balance funding for rare cancers given that the human impact can be more limited but, if you do not provide support, nobody else might? He described how this debate between whether to spend most of the NCI’s funding on common cancers (eg. breast cancer) or more rare cancers (eg. angiosarcoma) has been a lively 30-year debate at the NCI but has now been largely reconciled. Indeed, cancer research has really embraced the problem of rare cancers because, in reality, all cancers are molecularly unique. For example, even common cancers may have a rare molecular driver. As a result, researchers many come up with a therapy that treats a rare cancer but also works for a certain subset of lung cancer: the therapy translates because the molecular drivers of the cancer are more important than the histological subtype. The NCI sometimes does face mild criticism that it does not spend enough on breast cancer, pediatric cancer, etc., but Dr. Sharpless emphasized how the NCI’s focus has been on patients with cancer, including rare cancers because they want to see progress for all patients with cancer.
References
American Society of Microbiology. Infographic Depicting Differences between PCR and Serological Testing. PBS, www.pbs.org/newshour/science/this-antibody-test-could-offer-a-clearer-picture-of-how-the-body-responds-to-covid-19. Accessed 26 May 2020.
“The First AIDS Drugs.” National Cancer Institute, ccr.cancer.gov/news/landmarks/article/first-aids-drugs. Accessed 26 May 2020.
Harvard Global Health and Leadership Conference 2020. Evensi, www.evensi.us/harvard-global-health-leadership-conference-2020-university/339237337. Accessed 13 June 2020.
Sharpless, Norman. “Perspectives from U.S. Government: A Conversation with Dr. Norman Sharpless.” Global Health and Leadership Conference, 24 May 2020. Speech.
Sharpless, Norman. 17 Oct. 2017. National Cancer Institute, visualsonline.cancer.gov/details.cfm?imageid=11682. Accessed 26 May 2020.
Thomas, Mark. NHS Staff in Theatre. The Guardian, www.theguardian.com/world/2020/apr/04/coronavirus-crisis-is-stopping-vital-cancer-care-doctors-say. Accessed 26 May 2020.