About a year ago, I took two HMX fundamentals courses on physiology and pharmacology from Harvard Medical School. These courses were incredibly fascinating and intriguing, not only because they helped to explain concepts typically taught in medical school to me as a high schooler but also because I was able to appreciate the underlying implications for health and wellness. From time to time, I want to share what I learned from these courses with you all, and today we will be diving into acids and bases as a part of the HMX physiology course.
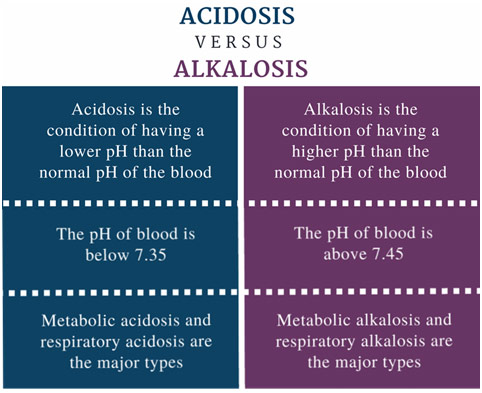
Enzymes are proteins in our body that allow our cells to control chemical reactions, but they can only operate at very narrow ranges of pH before they start to denature and lose their function. As a result, the body has to constantly maintain a certain pH (7.40 [7.36-7.44]), which is facilitated by both the respiratory and urinary systems. Let’s take an example to demonstrate how these systems influence pH in the body. Imagine that you just got terrible news, and this news causes you to feel quite anxious. As a result, you begin to breathe deeper and deeper, and faster and faster. These phenomena is known as hyperventilation and, when we hyperventilate, we eliminate more carbon dioxide than we produce: you have less carbon dioxide in your body. The following equation shows how carbon dioxide (CO2) is related to other molecules in the body, such as carbonic acid (H2CO3), a bicarbonate ion (HCO3–), and a hydrogen ion (H+).
CO2 + H2O ⇔ H2CO3 ⇔ HCO3– + H+
If you have taken a chemistry class at some point, you likely remember that pH is a function of how many hydrogen ions are present. Going back to the hyperventilation, when you have less carbon dioxide in the body, the reaction above shifts towards the left-hand side to “make up” the missing carbon dioxide. Of course, if you shift to the left, you must have shifted away from the right and thus away from the hydrogen ions. Given all that, when you hyperventilate, your pH increases (i.e., becomes more alkaline); these phenomena are known as respiratory alkalosis. When you hyperventilate, you might feel some tingling in your mouth and have your hands cramp up because, at a higher pH, calcium is less likely to be ionized and thus causes neuromuscular system malfunctions. Hypoventilation, taking too few breaths, would cause the opposite problem as too much CO2 is retained, which causes pH to decrease in what is called respiratory acidosis. The respiratory system can help us maintain our body’s pH, but it can simultaneously take us away from the narrow range that is compatible with life.
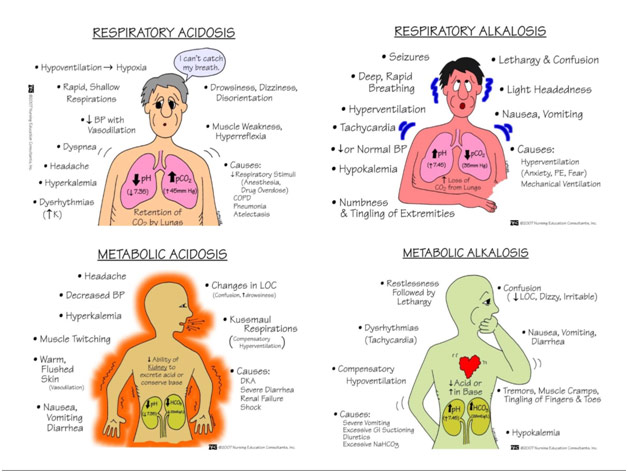
The thing is that our body is constantly making acid through metabolism. Metabolism can either be aerobic (with O2) or anaerobic (without CO2). Proteins, fats, and carbohydrates can all be metabolized anaerobically, releasing sulfates/phosphates, lactate, and ketones, respectively, as well as hydrogen ions (metabolic acid). Carbohydrates can also be metabolized aerobically with the waste product produced of carbon dioxide, which we have already shown is related to pH (respiratory acid). The acid we produce aerobically is typically eliminated by the lungs while the acid we produce anaerobically is typically eliminated by the kidneys.
As we have mentioned before, acidity is a function of the concentration of hydrogen ions, which are also known as protons. Protons combine with bicarbonate in the bloodstream, which makes carbonic acid, which dissociates into carbon dioxide and water as if the above reaction happened in reverse. The carbon dioxide that diffuses into the tissue of the kidney and ultimately into the renal tubular cells, where it once again becomes a proton and bicarbonates. The proton is secreted into the tubule and out through the urine while the bicarbonate ion is preserved, going back into the bloodstream. Of course, the kidneys operate on a different timescale than the lungs: acid is excreted in seconds by the lungs but days by the kidneys. However, in the case of chronic respiratory alkalosis, a patient might have metabolic compensation where the kidneys secrete out fewer hydrogen ions in order to maintain systemic pH. However, as problems with breathing can cause problems with pH, so too can problems with the kidneys cause problems with pH. If the kidneys are not functioning and are not excreting out metabolic acid, the patient might get metabolic acidosis because the hydrogen ions are not able to be secreted out of the body through the urine. If the kidney retains too few hydrogen ions, however, you can get the opposite problem with metabolic alkalosis.
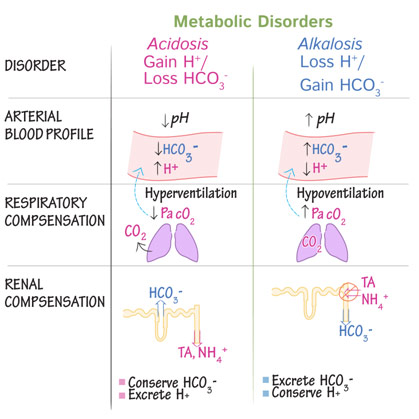
Compensatory Mechanisms for Acidosis and Alkalosis
To help check acid levels in a patient, a physician may sometimes order an anion gap blood test. Essentially, the anion gap test gives you a measure of the difference between your cations, positively charged ions, and anions, negatively charged ions. More specifically, you take your most commonly measured cations (Na+ and K+) and subtract your most commonly measured anions (HCO3– and Cl–). The normal anion gap is about 12-16 mEq/L. Given all that you know about the body, the fact that the normal anion gap is not 0 may seem strange because the number of cations must equal the number of anions for the whole system to be electrically neutral. However, this gap is accounted for by the negatively charged albumin protein.
In any case, a low anion gap is incredibly rare, but, if there is a high anion gap, you may have acidosis. More specifically, a high anion gap may indicate acidosis because of a number of conditions, denoted by the acronym MUDPILES. M – methanol, U – uremia (elevated nitrogenous wastes in the blood), D – diabetic ketoacidosis (elevated ketones in the blood), P – propylene glycol, I – isoniazid (antibiotic), lactic acidosis (elevate lactic acid in the blood), E – ethylene glycol, and S – salicylates (elevated salicylic acid in the blood). Understanding the root cause of the acidosis is incredibly important to caring for the patient and ensuring that the patient’s pH is kept safely in the narrow range needed for the body to function.
Understanding physiology is so vital because, by understanding how the body normally functions, we can begin to understand abnormal states and how we can restore a person’s health. From respiratory alkalosis/acidosis to metabolic acidosis/alkalosis, understanding acids and bases in the body is incredibly important to understanding how our body maintains homeostasis with pH.
REFERENCES
Acidosis & Alkalosis – Causes and Compensations. Physiology Flashcards, www.drawittoknowit.com/course/physiology/glossary/pathophysiologic-disorder/acid-base-disorders. Accessed 25 Sept. 2020.
Hobbs, Ashley. Respiratory/Metabolic Acidosis Vs. Alkalosis. Pinterest, www.pinterest.com.mx/pin/6122149480012674/?nic_v2=1a7hmGKFW. Accessed 25 Sept. 2020.
Lake. Difference between Metabolic and Respiratory Acidosis. Pedia, pediaa.com/difference-between-metabolic-and-respiratory-acidosis/. Accessed 25 Sept. 2020.
Schwartzstein, Richard. “HMX Fundamentals Physiology.” Lecture.
Wilczynski, Cory. “Anion Gap.” Edited by Eric Stars. Medscape, 15 Nov. 2019, emedicine.medscape.com/article/2087291-overview#a2. Accessed 25 Sept. 2020.