A few years ago, I took a Stanford CME Course on antibiotics and outpatient infections. Antibiotic misuse is one of the largest issues confronting healthcare in this day and age, leading to the proliferation of dangerous antibiotic-resistant bacteria that modern medicine currently has no great tools against. There are 700,000 deaths around the world each year due to antibiotic-resistant infections, and some estimates suggest that 10 million people could die annually from antibiotic-resistant bacteria by 2050. If such a trend is observed, getting a surgery or even going to the hospital would be incredibly risky because of the prevalence of surgical-site and hospital-based infections. Understanding the antibiotic crisis and management of common outpatient infections thus takes on special importance to avoid this crisis that could drastically blunt the way that healthcare is delivered.
Imagine that you are a primary care physician. A patient comes into your office with a headache, a sore throat, and a stuffy nose. Examining the patient and asking him some questions, you ascertain that the patient does not have a fever and has not had any chills. Recommending the patient take it easy for a couple of days and let the virus run its course, you begin to leave, but then the patient demands antibiotics. You try to explain that you are pretty sure that they have a virus, and antibiotics do nothing for bacteria. The patient is insistent, harping on your “pretty sure” and indignantly asserting that they are going to tell everyone how their doctor refused to treat them. Perceiving the patient’s demands and your time constraints, you finally relent and comply, prescribing the patient antibiotics so that they are satisfied.
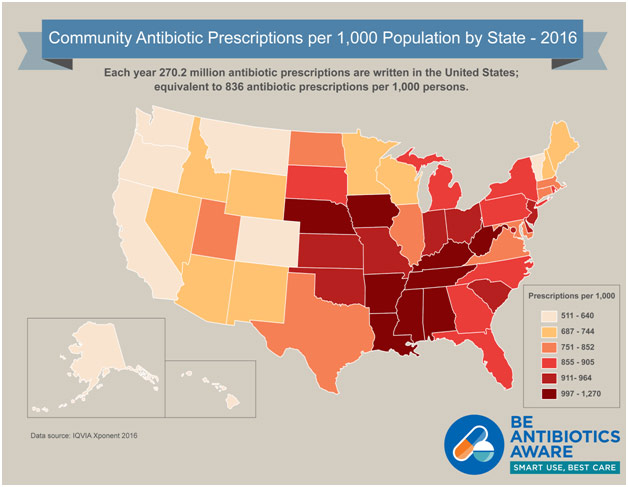
Antibiotic Overprescription by State
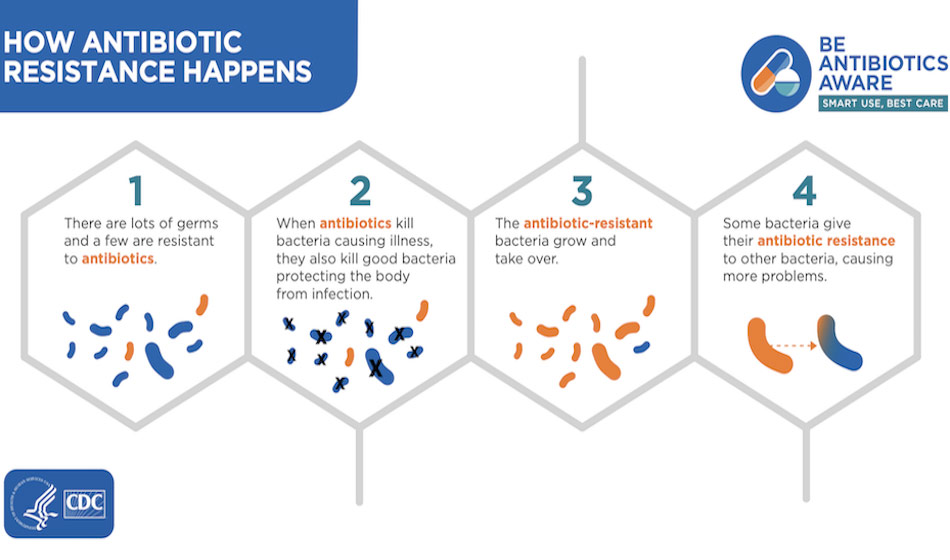
Unfortunately, this scenario, or a version thereof, happens far too often. Every year, twelve million unnecessary antibiotic prescriptions are written for upper respiratory tract infections. Children with common colds are prescribed antibiotics 44% of the time, with upper respiratory tract infections 46% of the time, and bronchitis 75% of the time. These antibiotics do nothing for the patient’s viral infection; they are prescribed even though the patient does not need them. However, this overuse does not go unpunished. The antibiotics will kill any bacteria that is present but typically not 100% of the bacteria, leaving the 0.1% or so that happens to be resistant to proliferate because all of its competition has been done away with. Prescribing an individual patient antibiotics for a viral illness is not devastating in and of itself; it is the cumulative effect that has the potential to create multidrug resistant bacteria that medicine has no weapons against.
Physicians thus need strategies to avoid being pressured by their patients into prescribing antibiotics for viral illnesses. One helpful acronym is GET SMART. The G stands for gaining trust through both verbal and nonverbal means, appearing professional, making eye contact, and giving clear instructions. If the patient trusts that you care about them and want to do the best for them, they will be more likely to agree with your recommendation of no antimicrobial therapy.
The E stands for empathizing through validating your patient’s concerns and building rapport, again so that the patient recognizes you are looking out for their best interest. The T stands for taking time to elicit expectations, which is critically important so that you can understand what the patient specifically wants to get out of their visit. The S stands for sharing findings, which is important so that you can reassure the patient and make the patient feel like they are a part of the decision-making process. The M stands for making specific diagnoses because vague, dismissive terms like “just a viral illness” reduce the patient’s confidence that you know what is wrong with them while clear, rapid diagnoses do the opposite. The A stands for articulating next steps, which allows you to inform the patient on what they can expect with their illness and what the contingency plan might be if the patient gets worse. Finally, the T stands for treat: you do not want to send the patient home with nothing to show for the visit, instead giving prescriptions for symptomatic relief.

Ways Patients Can Combat Antibiotic Resistance
There are a few common outpatient infections for which it is helpful to delve into with this context. Let’s start with acute bronchitis, which involves the patient coughing for at least 5 days duration, and the cough may persist for several weeks. The patient may have throat discomfort or cold symptoms, may produce sputum in their saliva, and may be wheezing significantly, although the patient does not have a fever. Viruses cause about 90% of acute bronchitis cases, which means that antibiotic therapy is neither effective nor recommended for acute bronchitis as antibiotic therapy is associated significantly with the risk of adverse effects.
Now, let’s move on to abscesses, a collection of pus that has built up. A majority of infections are caused by the Staphylococcus aureus bacteria, but the role of antibiotics in the management of abscesses is currently uncertain because there is a lack of compelling data either way.
Currently 75% of simple abscesses are treated with simple incision and drainage alongside a 10-day antibiotic course, but there may not be a need for antibiotics. This is especially true given that about 50% of abscesses in the U.S. are due to community-associated methicillin resistant Staphylococcus aureus, demonstrating how an antibiotic-resistant bacteria can spread outside the hospital. Small abscesses can typically be managed with warm compresses while larger ones can be incised and drained. This case study is not to say that there is no role for antibiotics in treating abscesses but to encourage caution in its use, especially when there is adequate source control of the infection and the patient does not have other risk factors such as a fever.
Finally, let’s cover asymptomatic bacteriuria (ASB). You take a urine culture of a patient and find that the patient has 105 colony-forming units per mL of E. coli bacteria; however, the patient does not have any symptoms of a urinary tract infection, which is much more complicated. This type of bacteria is known as an ASB as the patient does not have dysuria (pain while urinating), increased urgency/frequency of urination, pain in the region, nor a fever. The evidence here is very clear that antibiotics do not help the ASB nor they prevent urinary tract infections. As a result, unless the patient is part of two special populations (pregnant or getting a urologic procedure), antibiotics are not warranted.
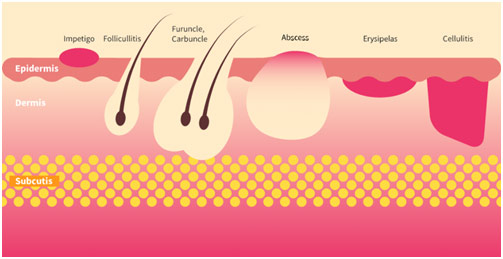
Different Types of Skin and Soft Tissue Infections (SSTI)
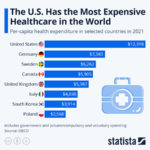
The world’s antibiotic crisis is not something that can be explained by a few malicious actors; it is a product of individual actions by physicians. Giving antibiotics to a single patient who does not really need it may not deleteriously impact that patient, but it is the cumulative effect of overprescribing antibiotics that has the potential to wreak havoc on our healthcare systems. Developing new antibiotics is a costly and time-consuming task: we cannot rely on research to bail us out of this crisis, especially considering that pharmaceutical companies have largely shifted away from investing in this area. Only through systemic change to limit the overuse of antibiotics and thus the prevalence of antibiotic-resistant bacteria can help alter the course of medicine.
REFERENCES
https://www.theguardian.com/society/2017/oct/13/antibiotic-resistance-could-spell-end-of-modern-medicine-says-chief-medic
https://online.stanford.edu/courses/som-ycme0020-prescribe-or-not-prescribe-antibiotics-and-outpatient-infections-cme
https://journals.lww.com/em-news/fulltext/2002/02000/patients_who_demand_antibiotics_and_the_doctors.12.aspx
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4240113/








Your logic should be accepted as the rule when it comes to this topic.
I loved your blog article. Really looking forward to read more. Want more. Kerrill Lenard Norbie
Glad to see that this site works well on my Google phone , everything I want to do is functional. Thanks for keeping it up to date with the latest. Daisie Clarence Marsha
I cannot thank you enough for the article post. Thanks Again. Awesome. Eda Torin Joeann
Hello. excellent job. I did not imagine this. This is a great story. Thanks!
I would like to convey my passion for your generosity for individuals that need help on this matter. Your personal dedication to getting the solution all-around came to be wonderfully interesting and have usually made most people just like me to achieve their objectives. Your amazing insightful suggestions signifies a whole lot to me and a whole lot more to my mates. Thank you; from all of us.