A few months ago, I had the opportunity to visit the Duke University Research Lab at Medanta Hospital in Haryana, India. Dr. Pooja Sharma, the director of the Medanta Duke Research Institute, and Kuldeep Chauhan, a project manager there, very kindly gave me a tour of the facilities and told me of some of the projects they were doing in collaboration with Duke University. The experience was spectacular as it gave me some insight to the amazing medical research that is being performed around the world.

Mr. Chauhan (left) and Dr. Sharma (right) at MDRI
MDRI is one of two labs around the world in joint collaboration with the main lab at Duke University and is approved by the drug controller general of India to do early phase clinical trials, or, in other words, proof of concept studies. The benefit of having these three different labs (one in India, one in Singapore, and one in the USA) is that they can get 3 different gene pools: Indian, East Asian, and Caucasian. MDRI is unique because very few other hospitals in India have a research lab associated with them because it is hard to structure such a program and get past all the regulatory hassles.
The main focus of MDRI is to do clinical trials, and they have done some from stem cells for heart disease to stroke to spinal cord injuries to diabetes. The steps followed at MDRI for these clinical trials go as follows. First, in collaboration with Duke, the study proposal, as well as consent forms and source records, are prepared. Then, based on the area of expertise, the study team at MDRI is identified. This team consists of a principal investigator, project manager, pharmacist, etc. The dossier of the study is then submitted to the ethics committee for review and approval. The ethics committee does a risk-benefit analysis of the experiment and assesses the protocol to see if it is scientifically sound and ensures the participant’s safety. The ethics committee consists of lawyers, pharmacologists, nurses, and doctors as well as some lay-people. To prevent a conflict of interest, the chairperson of the ethics committee is completely unassociated with the institution. If the ethics committee approves the study, the research team does a dry run without patients to review the steps of the protocol. Then, the actual study is conducted on patients in the hospital who have signed a consent form. Based on the study requirements, the participants may need to have their blood work, ECG, x-ray, echocardiogram, etc. done to determine eligibility for participation. The patients who participate in the study see their travel, treatment, and other hospital expenses become free, and they get some additional reimbursement as well. The study team gives a weekly update to Duke, but at any point of time, the Duke team can come to MDRI to check and assess the study. Sometimes the study may be terminated if the investigator or the Duke sponsor recognizes that there is more harm than good being done to the patient with the study.
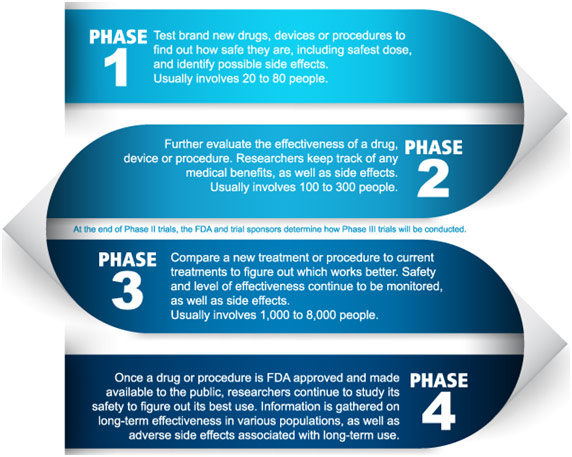
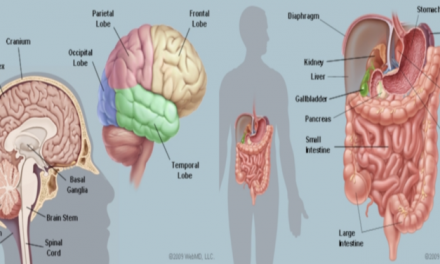
Phases of a Clinical Trial
Mr. Chauhan was kind enough to give me a tour of the facilities of MDRI. We saw the screening era where the researchers determine if the patient should be excluded or included in the trial, checking vitals among other things. He then showed me the group consent area where prior to the study participation, all participants sign a consent form stating that they know what activities will be performed and for what purpose they will be performed. For instance, they may consent to the researchers taking only a certain number of blood samples each day. During the study period, the patients stay in the 63-bed facility, which, when not used by MDRI, can be used for normal hospital functions. Mr. Chauhan also showed me their metabolic kitchen where food is made in accordance with the FDA’s criteria as well as the pharmacy area where researchers can access the controls as well as the drugs they are researching. In this pharmacy area, there are various fridges all set at different temperatures based on the needs of the various drugs. A security team comes in every 2 hours to make sure that everything is okay, and there are alarms that sound if the temperature in the fridges changes from the baseline. Drugs are often quite temperature-sensitive, so staying within the narrow range they can tolerate is absolutely paramount. Since MDRI does a great deal of PK/PD studies, they also had a room where they process and store blood samples from the study participants. PK/PD studies, or pharmacokinetics and pharmacodynamics, seek to understand “what the body does to the drug” and “what the drug does to the body” respectively. One project that they were currently storing blood samples was a cardiometabolic study with more than 380 participants, designed to “identify novel metabolic markers” for CAD (coronary artery disease) and T2D (type II diabetes) in the Indian population.

MDRI Cardiometabolic Study
MDRI is also working on amazing projects like a chicken guinea vaccine that is first in man, an immunotherapy project using dendritic cells for cancer, and looking at yoga and the ancient Indian healing science Ayurveda through randomized controlled trials to evaluate their possible effectiveness. As hospital-acquired infections are a problem worldwide, MDRI is additionally working on an app to create an antibiogram that includes different bacterial infections and the drugs they are resistant to with the information changing in real-time. The basis of this app is looking at resistance patterns in the hospital to allow the doctor to prescribe the right antibiotic more accurately. Another aspect of MDRI’s work involves a tissue repository for cancer, which is designed, in part, to give the institution some commercial independence. They have received funding to look for biomarkers and different genetic patterns from breast and parathyroid cancerous tissues that cause drugs do not always work as anticipated. The hope of this project is to create better-personalized medicine.
MDRI also has extended its efforts towards public health like with Mission Stop Dengue and TB Free Haryana. Mission Stop Dengue aims to combat the mortality of the vector-borne disease known as dengue fever, which is specifically spread by mosquitoes. Through education on prevention, early diagnosis, and effective care, Medanta has started an effective campaign to fight this disease. MDRI is actually working on biodegradable sites that will kill the mosquito vectors without causing ecological problems. TB Free Haryana, meanwhile, seeks to help improve the diagnosis of tuberculosis, a respiratory infection that is underdiagnosed in rural India and Haryana in particular. Interestingly enough, one in four TB cases in the world finds their home in India, making this an urgent public health issue in the nation. Medanta has sent out multiple x-ray vans into underserved parts of Haryana to assist with diagnosis and start treatment immediately with the goal of ending TB in Haryana by 2020. MDRI is currently working with Delhi University on an AI that can determine if the patient’s X-ray shows that they have tuberculosis or not so that the diagnosis can be even faster and even more accurate.

Mission Stop Dengue
TB Free Haryana
MDRI’s community emphasis can also be seen with their support of the research of other organizations like CanKids…KidsCan, which works to address pediatric cancer in India, and InPOG (Indian pediatric oncologist), which only recently created their own R&D department. MDRI has also supported ORDI (Organization for Rare Diseases in India) to help create rare drug policy and develop orphan drugs, which are generally not commercially viable because of their selective focus. Lastly, MDRI emphasizes community outreach regarding medicine because as Dr. Sharma told me, research will only truly begin in India when Indian patients want it. Thus, MDRI did the PARTAKE study, which stands for Patient Awareness of Research and Therapeutic Advances for Knowledge and Empowerment. The goal was to determine how much does the Indian population actually knows about clinical research. In short, they found that while the Indian public “supports clinical research in general,” the public “exhibits some distrust in the conduct and reporting of clinical trials.” MDRI has thus worked on helping improve education on research in Haryana and India in general.

CanKids…KidsCan

ORDI
The ability to go around MDRI with Mr. Chauhan and learn more about the various projects which MDRI has worked on and is currently working on from Dr. Sharma was nothing short of remarkable. I learned so much about the medical research that is being done at MDRI as well as the amazing community and public health outreach that Dr. Sharma and her team has done here. The research that is being done worldwide is so remarkable and advanced that medicine will be nearly transformed when I start practicing in some 10-15 years.
REFERENCES
But, Tal, et al. “PARTAKE Survey of Public Knowledge and Perceptions of Clinical Research in India.” Plus One, 16 July 2013, journals.plos.org/plosone/article?id=10.1371/journal.pone.0068666. Accessed 19 Mar. 2019.
“Cancer-survivor’s Documentary Features NGO Working for Children Suffering from Deadly Disease.” The Indian Express, 7 July 2015, indianexpress.com/article/good-news/a-cancer-survivors-tribute-to-kolkata-brave-hearts/. Accessed 19 Mar. 2019.
DLF Foundation Partners with Medanta in ‘Mission Stop Dengue’. News Mantra, newsmantra.in/component/tags/tag/1736-mission-stop-dengue. Accessed 19 Mar. 2019.
Mission TB Free Haryana. Medanta, www.medanta.org/tb-free-haryana/. Accessed 19 Mar. 2019.
Organization for Rare Diseases India. Race for 7, racefor7.com/sponsors/. Accessed 19 Mar. 2019.
“Our Work.” Duke Global Health Institute, globalhealth.duke.edu/projects/medanta-cardiometabolic-disease-study. Accessed 19 Mar. 2019.
Pezzullo, John. “Pharmacokinetics and Pharmacodynamics (PK/PD Studies).” Dummies, www.dummies.com/education/science/biology/pharmacokinetics-and-pharmacodynamics-pkpd-studies/. Accessed 19 Mar. 2019.
What Are the Phases of Clinical Trials? 19 May 2015. Emory Healthcare, advancingyourhealth.org/highlights/2015/05/19/understanding-clinical-trials-phases/. Accessed 19 Mar. 2019.