In 1922, Sir Frederick Banting made the revolutionary discovery of insulin as a treatment for diabetes. He sold his patent for $1, saying “insulin belongs to the world, not me.” He refused to profit from such a transformative, life-saving treatment. Nearly 100 years later, patients with diabetes are forced to shell out, on average, $450 for insulin a month, and, as a result, one in four diabetics must ration their insulin with potentially devastating consequences to their health. Similarly, the price of EpiPens, a lifesaving medication for anaphylactic shock, has skyrocketed, going from $100 in 2007 to $608 in 2016. Unfortunately, soaring prices for these and other drugs are a staple of the US healthcare system, and, in this blog post, I want to delve into this aspect of America’s pharmaceutical crisis, explaining both sides of the issue, a tug-of-war between access and innovation.
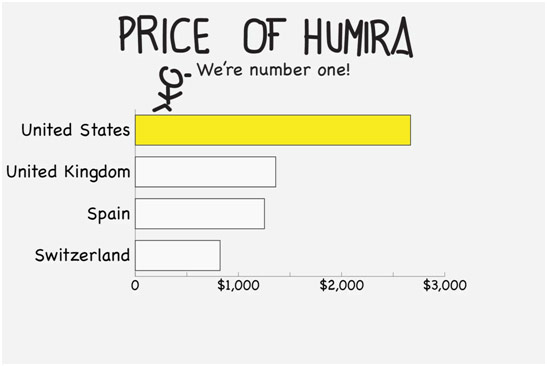
Cartoon Depicting Humira Prices around the World
It is important to first establish how incredibly difficult it is to bring a drug to market. Only 1 out of every 40,000 potential compounds make it to market, and bringing a drug to market costs about $2.6 billion. In the US, it is so challenging to get a drug approved and in the hands of consumers largely because of the FDA’s stringent regulations.
Drug discovery generally begins with the identification of an active ingredient or a pharmacologic compound, which subsequently undergoes preclinical testing. Once that testing is over, clinical trials may begin: Phase I assesses the safety of the drug in a small number of volunteers, Phase II tests for dosing and efficacy on several hundred patients afflicted with the disease, and Phase III tests for effectiveness and adverse effects in several thousands of patients. After Phase III trials finish, the pharmaceutical company can get approval to use the drug for widespread use, something that, on average, takes about 12 years to get to from drug design and discovery. Phase IV trials are simply monitoring consumer use and long-term effects.
With this rigorous process comes high attrition rates at every step. At the preclinical stage, 60% of candidates fail. At the Phase I stage, 30% fail. At the Phase II stage, 65% fail. At the Phase III stage, 75% fail. An enormous amount of time and money is needed to even attempt to bring a drug to market, and, most of the time, it all goes down the drain. In the astonishing case that a drug actually makes it to market, only one-third of approved drugs recover their production costs. At the end of the day, pharmaceutical companies are companies, not charities. They need to price drugs so that they recoup the expenses of not only all their unprofitable approved drugs but also all the candidates that fell into the abyss, never even making it to market. For pharmaceutical companies to continue making medical advances, they need to price their drugs to make a profit and make their investors stay happy. After all, investors are often simply looking for the largest return on investment. It is more societally beneficial if investors put their money into creating new drugs than a mindless smartphone app, but, if a drug companies’ profit decreases, investors’ money will flow to the app, away from scientific advances and lifesaving innovation.
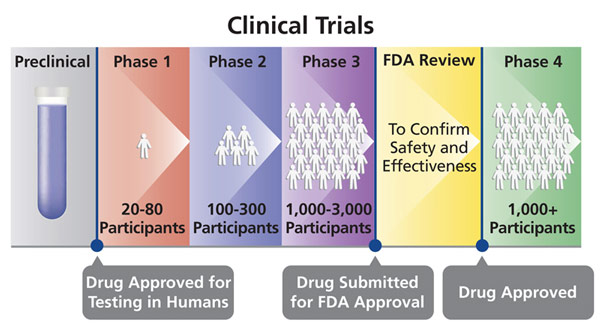
Stages of a Clinical Trial
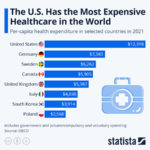
However, US drug prices are not exorbitantly high simply because the drug approval process is riddled with obstacles: we also do not reap the benefits of scale in the US. For everything, the more you buy, the cheaper you get it. With Americans scattered among the numerous private insurances, there is no central governing body that can effectively negotiate down drug prices. Medicare, the closest the US has to a national healthcare system, buys drugs for 60 million Americans, so, in theory, they should be able to get a more reasonable price from drug companies. However, the Medicare Modernization Act makes government negotiations with drug companies illegal, which certainly has grave consequences. For example, Medicare pays 40% more for drugs than the Veterans Health Administration, which can and does negotiate drug prices. On top of that, drug companies get to set their own prices, and Medicare must provide access to whatever drug the FDA deems safe, no matter if there already is a better and cheaper alternative. The result is that Americans pay the most for drugs in the entire world.
Thus, pharmaceutical companies negotiate with other countries’ central bodies to give them affordable drug prices and then charge as high as they need to in the US to still make a profit. This idea is known as cross-subsidization: basically, the US is paying for the research and cheaper access to drugs for the rest of the world. This is where the idea of importing drugs from Canada, which has a national healthcare system, comes in because US consumers could benefit from cheaper Canadian drug prices. If we take this idea to the extreme or suddenly the US federal government starts negotiating drug prices down, however, another issue arises. Without the US overpaying for drugs, the money put towards research and innovation would catastrophically drop. The US would have greater access to care at the expense of global drug innovation. The question is where do we balance access and innovation, patients of the present, and patients of the future.
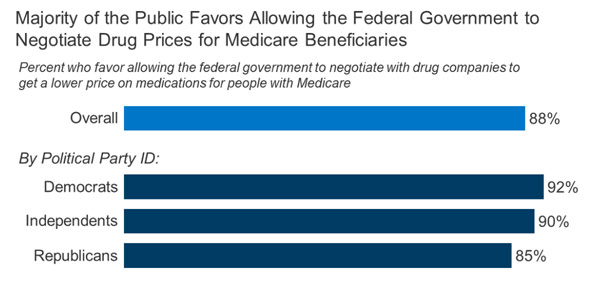
Widespread Support for Medicare Negotiating Drug Prices
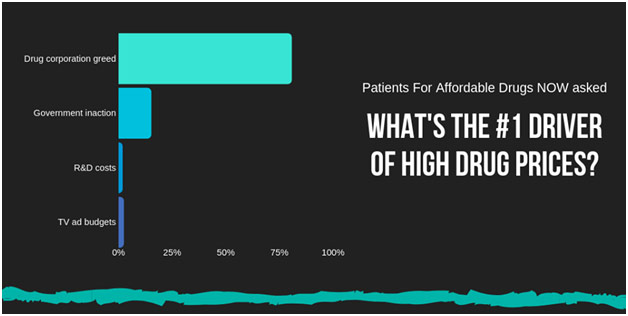
Presently, the situation has gotten quite abysmal in the US; the pendulum has swung too far in favor of innovation. Corporate greed perhaps explains more about high drug prices than anything else. Nine out of the top ten pharmaceutical companies spend more on marketing their products than on research, convincing consumers to get their doctors to prescribe them that medication, whether the patient needs it or not. Direct-to-consumer drug advertising is actually illegal in every other country but the US and New Zealand because it unnecessarily exaggerate demand for costly new drugs. Furthermore, the revenue from the top twenty most popular drugs in the US covers all the R&D costs for a drug company while still leaving $40 billion to spare for profit. Revenues from drugs not in the top twenty are just icing on the cake of profit. Negotiating down drug prices therefore do not necessarily chip at funds set for R&D because marketing costs and immoderate profits would be on the chopping block beforehand. In the words of former Merck CEO Raymond Gilmartin, “‘The price of medicines is not determined by their research costs. Instead, it is determined by their value in preventing and treating disease.’” As a result, it comes to no surprise that pharmaceutical companies raise prices on drugs that were invented decades ago to extraordinary extremes. They do it because they can because nobody is stopping them. Research does not determine drug prices; tantalizing profits determine drug prices.
We need to emphasize greater access to medication. Indeed, the human cost of exorbitant drug prices is evident even from my personal experiences. I shadowed endocrinologist, Dr. Nadia Hameed, a couple of years ago, and I saw her give drug samples to patients who could not afford their insulin. I saw patients cry in her office because of how expensive their lifesaving medication was. I saw patients who gambled with their lives every day, rationing their insulin so that it would last just a little bit longer. I saw patients who had set up GoFundMe pages to pay for the drugs that kept them alive. We fail patients when we price them out of their medicine
REFERENCES
https://mcgill.ca/channels/channels/news/affordable-life-saving-medicines-all-mcgill-adopts-global-access-licensing-principles-research-297381
https://www.vox.com/2019/4/3/18293950/why-is-insulin-so-expensive
https://www.usnews.com/news/articles/2016-09-21/mylan-head-defends-epipen-price-gouging-in-capitol-hearing
https://www.vox.com/science-and-health/2016/11/30/12945756/prescription-drug-prices-explained
https://www.theatlantic.com/health/archive/2019/03/drug-prices-high-cost-research-and-development/585253/
https://www.reuters.com/article/us-pharmaceuticals-advertising/u-s-doctor-group-calls-for-ban-on-drug-advertising-to-consumers-idUSKCN0T62WT20151117